Biomedical Engineering Reference
In-Depth Information
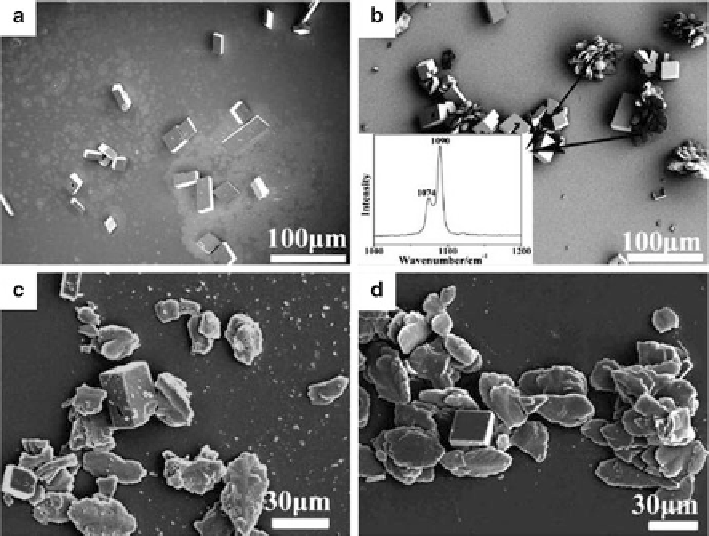
Fig. 6.16
SEM images of the CaCO
3
particles collected under a DPPC monolayer at different
surface pressures after 9 h of reaction: (
a
)0,(
b
) 2 (the inset displays a Raman spectrum of the
floret-like crystals), (
c
) 25, and (
d
) 40 mN/m. The irregularly shaped crystals in (
c
)anddare
the same as the floret-like crystals in b except that the petal-like crystals have been detached via
sonication. The very small debris arising from the detachment process can be clearly seen in (
c)
.
(Reproduced from [
104
], Copyright © 2010, American Chemical Society)
bacteria [
100
] and the mineralizing tissues of vertebrates [
101
-
103
]. Yang and
co-workers reported the mineralization processes of calcium carbonate under a
phospholipid monolayer at the air-water interface (Fig.
6.16
)[
104
]. The ACC
precursor was obtained by the Kitano method. It was unstable and thus transformed
into the inter-mediate vaterite. Then, the vaterite was transformed into the most
thermodynamically stable calcite crystals eventually. This experiment result can
be explained by the empirical Ostwald-Lussac law, which may reflect the biomin-
eralization processes of cellular membranes. Furthermore, the spontaneity of the
transformation from vaterite to calcite was found to depend sensitively on the degree
of monolayer compression instead of the escape rate of the CO
2
, implying that
surface tension is a dominant factor in the transformation. This work has clarified
the crystallization procedure of calcium carbonate under phospholipid monolayer
and thus may further the understanding of the biomineralization processes induced
by cellular membranes.