Biomedical Engineering Reference
In-Depth Information
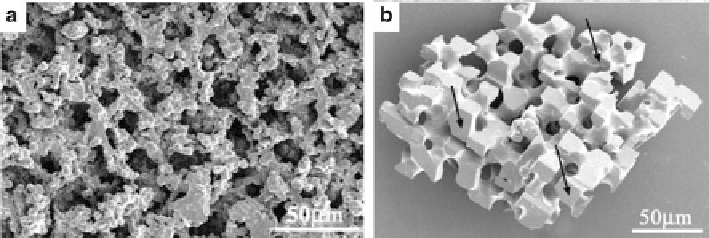
Fig. 6.10
Calcium carbonate precipitated in the polymer membrane. (
a
) Polycrystalline particle
precipitated from 0.4 M reagents, (
b
) templated single crystal of calcite precipitated from 0.02 M
solutions. (Reproduced from [
82
], Copyright © 2002, Wiley)
single crystals with complex form can be produced without using additives, by
external imposition of morphology (Fig.
6.10
). Calcium carbonate was obtained
in a polymer membrane, which had a same morphology to a sea urchin skeletal
plate. The polymer membrane was templated by a sea urchin plate and was
produced by dipping a plate in a polymer monomer solution, curing the polymer,
and finally dissolving away the calcium carbonate to obtain the polymer replica.
Precipitation of CaCO
3
in the membrane was received by placing the membrane
between two half U-tube arms, and filling them with Na
2
CO
3
and CaCl
2
solutions
respectively [
82
]. After a designated time, the membranes were removed from the
U-tube set-up, washed with water, and allowed to dry at room temperature for
1 day. Removal of the membrane by heating (at 773 K for 45 min) yielded the
particles of calcium carbonate that had been precipitated in the membrane [
82
]. The
experimental results demonstrate that the single crystals of calcite with sponge-like
morphologies were readily produced by precipitation within a suitable constrained
volume under controlled nucleation and growth conditions. They also investigated
the morphological control of single crystals by using the biological mechanisms
as inspiration [
84
]. Calcite was obtained within the cylindrical pores of track-etch
membranes via an ACC precursor.
Mosaic single-crystal CaCO
3
thin films can be obtained on modified
poly(ethylene terephthalate) (PET) templates [
85
]. The thin films can be fabricated
on chemically modified PET template surfaces by the process of transformation of
ACC film into a crystalline phase in air. The experimental results confirmed that
single-crystal CaCO
3
growth patterns are dependent on the surface characteristics
of the PET template. Therefore, the nucleation and growth of ceramic films on
polymeric templates can be controlled by chemical modification of the polymeric
template surface, and by subsequent attachment of ionic polyelectrolytes.
Qi et al. reported a novel approach to obtain three-dimensional ordered macrop-
orous (3DOM) calcite single crystals by combining the amorphous-to-crystalline
strategy with the use of colloidal crystals as 3D templates [
86
]. The results
demonstrated that that it is feasible for ACC to form single calcite with a