Biomedical Engineering Reference
In-Depth Information
1.0
3
8%
0.9
0
13%
0.8
5
.
8
%
-3
0.7
-6
0.6
-9
32%
0.5
TG
DTA
-12
0.4
-15
100
200
300
400
500
600
700
800
Temperature (
o
C)
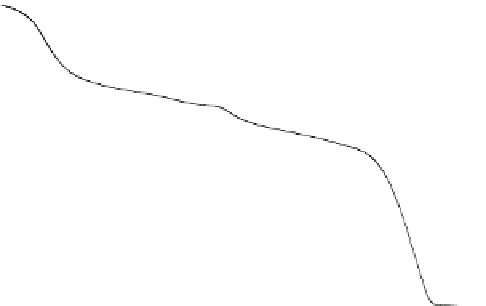
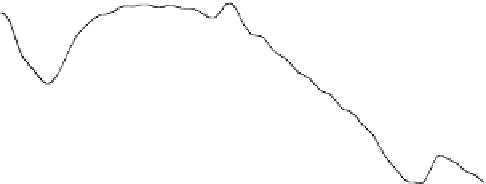
Fig. 6.3
Thermogravimetric and differential thermal curves of magnesium amorphous calcium
carbonate (Mg-ACC) powder. (Reproduced from [
53
], Copyright © 2010, Royal Society of
Chemistry)
The possible structure of the obtained Mg-ACC can be defined as Mg
0.15
Ca
0.85
CO
3
H
2
O
0.85
(Fig.
6.3
). The molar ratio of Mg
2C
:Ca
2C
:CO
3
2
and the concentration
(CaCl
2
,Na
2
CO
3
,andMgCl
2
) play important role in the Mg/Ca molar ratio of the
obtained Mg-ACC. In particular, the Mg-ACC can be preserved for over one year
without crystallization by either storing its dry powder at
5
ı
C or storing it in
ethanol at 5
ı
C. The access of Mg-ACC nanoparticles in large scale would be useful
for further biomineralization study and industry applications.
6.2.1.3
The Na
2
CO
3
-PAA-CaCl
2
Aqueous Reaction System
The nanosegregated amorphous composites consisting of CaCO
3
and an organic
polymer can be prepared by this method (Fig.
6.4
)[
54
]. The thin and bulk film
of the amorphous composites possessed stability and transparency. The amorphous
composite in the bulk state was formed by mixing the two solutions. The Na
2
CO
3
(0.1 M) solution was poured into the precursor solution containing polyacrylic
acid (PAA) (0.1 M) and CaCl
2
(0.1 M) at room temperature. A white precipitate
gradually appeared upon mixing of these solutions. The obtained product was
collected after 1-2 h by centrifugation and then the paste-like translucent material
was dried at room temperature. When the reaction took place without the PAA, the
ACC can also be obtained [
55
].