Biomedical Engineering Reference
In-Depth Information
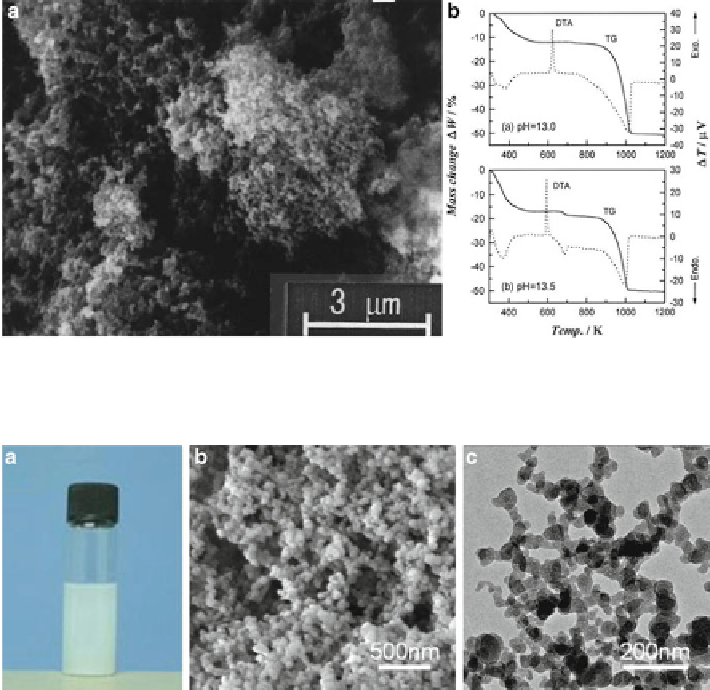
Fig. 6.1
(
a
) A typical SEM image of dehydrated ACC. (
b
) Typical TG-DTA curves for ACC.
(Reproduced from [
52
], Copyright © 1998, Elsevier)
Fig. 6.2
Magnesium amorphous calcium carbonate (Mg-ACC) obtained by rapid mixing of
the solutions (CaCl
2
,Na
2
CO
3
,andMgCl
2
). (
a
) the Mg-ACC powder dispersed in ethanol
homogeneously, (
b
) Scanning electron microscopy (SEM) images of the obtained Mg-ACC, (
c
)
Transmission electron microscopy (TEM) images of the obtained Mg-ACC. (Reproduced from
[
53
], Copyright © 2010, Royal Society of Chemistry)
ACC almost contains magnesium, which is presented in large quantities in seawater
(about 50-60 mM Mg
2C
, relative to the 12 mM Ca
2C
). Therefore, The Mg-ACC
plays a key role in the biomineralization of CaCO
3
.
In
L
1
,25mL)and
a
typical
experimental
procedure,
CaCl
2
(0.1
mol
6H
2
O (0.5 mol L
1
, 5 mL) were mixed with mechanical stirring to obtain
mixed solutions. Then, a Na
2
CO
3
solution (0.1 mol L
1
, 25 mL) was added rapidly
to the mixed solutions with mechanical stirring at an ambient temperature of
25
ı
C. The precipitated colloidal phase was filtered immediately and washed
with ethanol. The precipitates were dried in a vacuum desiccator for 1 day
(Fig.
6.2
)[
53
].
MgCl
2