Biomedical Engineering Reference
In-Depth Information
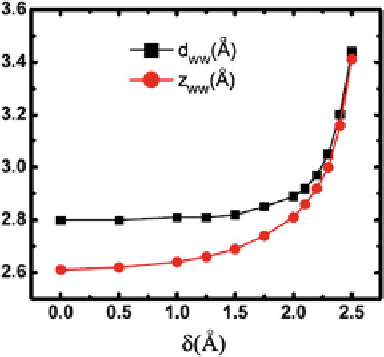
Fig. 1.7
The distance
between the two water
molecules near the
forced-atom
d
WW
and its
z
-projection
Z
WW
for different
ı (reprinted from [
129
].
Copyright 2008 American
Physical Society)
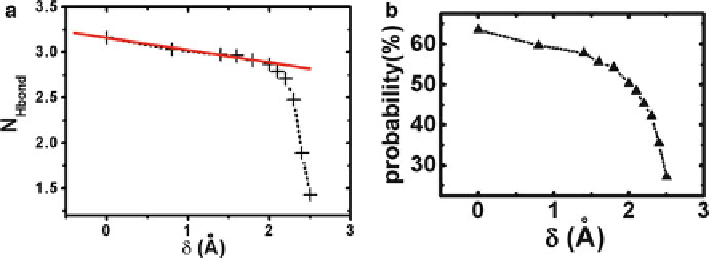
Fig. 1.8
(
a
) Average number of the hydrogen bonds inside the nanotube, the
solid line
,isa
linear fit when ı<2.0 A, and (
b
) the probability of the hydrogen bond formed by those two
water molecules neighboring the forced-atom for different ı (reprinted from [
40
]. Copyright 2005
American Chemical Society)
in distance between two water molecules near
P
can be shown by the probability of
the hydrogen bond formed by those two water molecules neighboring the forced-
atom (Fig.
1.8
). In the range of ı<2.0 A, the deformation is not strong enough to
perturb the hydrogen bonds chain inside the CNT, and the dynamics of water inside
the CNT (e.g., occupancy and net flux) keeps stable in this range. When ı
2.0 A,
the deformation is strong enough to break the hydrogen bonds chain, resulting in a
sharp decrease in water occupancy and net flux.
To explore the flipping behavior of water dipoles inside the CNT and its
sensitivity to the deformation, we define as the angle between a water dipole
and the nanotube axis, and
N
as the average angle of all the water molecules inside
N
N
the tube. Some
for different ı are shown in Fig.
1.9
. For the unperturbed CNT,
N
<50
ı
and 130
ı
<
N
falls in two ranges 15
ı
<
< 165
ı
most of the time, which is