Biomedical Engineering Reference
In-Depth Information
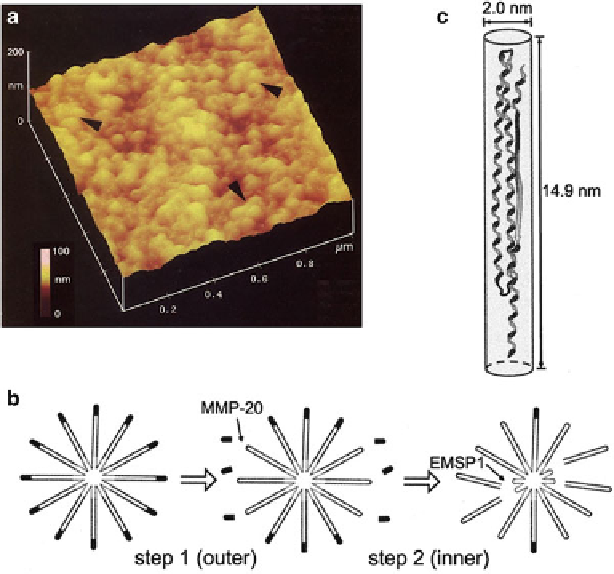
Fig. 5.2
(
a
) AFM image of porcine amelogenin gel (25 kDa (7.4%), 23 kDa (10.7%), and 20 kDa
(49.6%) amelogenins and smaller peptides (32.4%)) formed at 4
ı
C. Amelogenins assemble into
nanospherules 8-20 nm in diameter. (Reproduced with permission from ref. [
58
]) (Copyright 1999,
Elsevier) (
b
) Micelle model of 25 kDa amelogenin aggregate and proposed degradation by two-step
cleavage that provides space for crystal growth: (step 1) hydrophilic C-terminal domain is cleaved
by protease MMP-20; (step 2) cleavage by protease EMSP-1 releases 13 kDa fragments, leaving
behind smaller micelles containing 6 kDa fragments. (Reproduced with permission from ref. [
64
])
(Copyright 2007, Sage Publication) (c) Model structure of amelogenin molecule (porcine, 20 kDa
fragments) with three characteristic domains:
-sheet, polyproline, and random coil. (Reproduced
with permission from ref. [
77
]) (Copyright 2009, Wakaba Publishing Inc.)
“
amelogenin distribute outer surface of the nanospheres; (2) the nanospheres vary in
size depending on the molecular weight of the amelogenin and the pH, temperature,
and protein concentration of the solution [
22
,
58
,
59
]; and (3) the nanospheres
tend to form chains, which assemble into higher order structures, “microribbon,”
in vitro [
60
]. Structure of amelogenin is reviewed by several authors [
61
-
63
], and
also described in 2.2 of Chap. 4.
Another model structure based on the behavior of amelogenin in solution
has been proposed (Fig.
5.2
b) [
64
]. In this micelle model, the surfaces of the
25 kDa amelogenin micelles have a highly hydrophilic C-terminal domain, which
has a pattern of positive and negative charges. The micelles aggregate by ionic
interaction. Micelles of 20 kDa amelogenin without the C-terminus aggregate