Biology Reference
In-Depth Information
Ph
N
N
N
HN
O
M
N
N
NH
N
Ph
CB-I
M = Zn
CB-II
M = H,H
l
abs
= 650 nm
l
abs
= 675 nm
e
= 60,000 M
−
1
cm
−
1
e
= 80,000 M
−
1
cm
−
1
l
em
= 760 nm
l
em
= 760 nm
F
f
= 0.19
F
f
= 0.19
FWHM = 18 nm
Solvent: toluene
Ref. 202
FWHM = 21 nm
Solvent: toluene
Ref. 202
Chart 3.8 Structures and optical properties of chlorin
-
bacteriochlorin energy-transfer
dyads with tunable apparent Stokes shift.
solubilizing motif, that is, hydrophilic groups that are projected above and
below a macrocyclic porphyrinic plane.
204
Such a motif provides water sol-
ubility and prevents aggregation by sterically hindering the
p
-system. Thus,
chlorins equipped with swallow-tail substituents with phosphonic acids have
been reported, and they show good water solubility, no evidence of aggre-
gation, and good fluorescence quantum yields in water.
204
A similar ap-
proach has been developed by Vinogradov and coworkers, who prepared
benzoporphyrins with dendritic substituents. Dendrons attached to the
benzoporphyrin core possess peripheral hydrophilic groups (such as polyeth-
ylene glycol) and provide excellent water solubility, and crowded dendritic
substituents protect porphyrin from self-aggregation and interactions with
plasma proteins which may alter
the optical properties of
the
fluorophore.
173,174,205,206
An alternative strategy that has been developed to overcome the lack of
water solubility of porphyrinic compounds entails the encapsulation of
hydrophobic tetrapyrrolic macrocycles inside nanostructured capsules.
Therien and coworkers have embedded strongly coupled porphyrin arrays
into the polymersome membranes by cooperative self-assembly of diblock

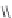











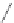















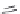










































Search WWH ::

Custom Search