Biology Reference
In-Depth Information
OR
RO
BuOOC
COOBu
BuOOC
COOBu
RO
OR
NH
N
N
N
M
N
HN
N
N
RO
OR
BuOOC
BuOOC
COOBu
COOBu
OR
RO
R = -(CH
2
)
2
-COOEt
P-I
P-II
P-III
l
abs
= 673 nm
M = H,H
M = Pd
e
= 46,000 cm
-
1
M
-
1
l
abs
= 696 nm
l
abs
= 673 nm
e
= 316,000 cm
-
1
M
-
1
l
em
= 676,748 nm
= 200,000 cm
-
1
M
-
1
e
l
em
= 923 nm
F
f
= 0.27
l
em
= 733, 810 nm
phosphorescence
= 0.04
Solvent: DMF
Ref. 171
F
F
f
= 0.45
Solvent: pyridine
Ref. 171
Solvent: pyridine
Ref. 171
Chart 3.5 Structures and optical properties of
representative red and near-IR
benzoporphyrins.
analogs for
in vivo
imaging have been less explored, though their optical
properties make them also suitable for those applications.
Porphyrins have a unique electronic structure that results in a complex ab-
sorption spectrum. Simple porphyrins (such as tetraphenylporphyrin) exhibit a
very strong (with
500,000 M
-1
cm
-1
)absorptionbandaround400nm,a
series of much weaker bands in the visible region (500-650 nm), and a very
weak absorption band in red spectral window (
e
650 nm).
170
Porphyrins pos-
sess also moderate fluorescence quantum yields (
0.1).
170
Although simple
porphyrins are not suitable for
in vivo
fluorescence imaging because of their
weak absorption in red/near-IR spectral window and their rather moderate
fluorescence quantumyields, several of theirmore elaborate derivatives exhibit
strong absorption and intense fluorescence in the red and near-IRregions. De-
rivatives with optical properties most promising for
in vivo
applications are
benzoporphyrins, strongly conjugated porphyrin arrays, and hydroporphyrins.
5.2. Benzoporphyrins
Extension of the aromatic systems in porphyrins by fusing the benzo (or
naphtho) ring at the pyrrolic positions causes a bathochromic shift and sub-
stantial intensification of the long-wavelength absorption band (with the












































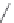




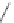
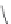










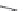





























Search WWH ::

Custom Search