Biology Reference
In-Depth Information
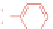
available squaric acid with electron-rich aromatic or heteroaromatic
compounds with simultaneous removal of the water formed during the
reaction (
Fig. 3.3
).
106,107
Two-step condensation affords nonsymmetrical
squaraines containing two different (hetero)aromatic groups.
106,107
Despite the favorable optical properties of squaraines, their application
for
in vivo
imaging suffers from two major limitations. While squaraines ex-
hibit often very high quantum yield of fluorescence in nonpolar solvents,
they extensively aggregate in polar organic solvents or in water and the ag-
gregation dramatically changes both absorption and emission properties:
broadens their absorption spectra and reduces significantly their fluorescence
quantum yields.
110
The electron-deficient hydroxyoxocyclobutene core of
squaraines is prone to reacting with nucleophiles, including those present in
biological media (especially thiols). The nucleophilic addition breaks the
electronic conjugation and the resulting product loses near-IR absorption
and fluorescence.
111
The breakthrough that has overcome these problems and opened the
door for the broad application of squaraines for
in vivo
imaging was the dis-
covery that the stability of squaraines can be significantly improved upon
formation of rotaxanes (for a review of the early development of fluorescent
A
O
O
O
R
1
R
1
R
2
R
1
R
1
R
2
R
1
n
-butanol/benzene
heating
+
N
R
2
N
2
N
R
2
HO
O
N
R
2
N
OH
O
O
B
R
3
O
O
O
N
R
4
R
1
R
1
R
1
R
3
R
4
(1) acid
(2) H
2
O/H
+
+
Cl
O
N
R
2
N
R
2
N
N
R
2
O
Cl
O
O
C
O
O
HN
NH
NH
2
O
O
R
R
R
R
R
R
O
O
N
R
N
+
+
N
R
N
Cl
Cl
O
O
NH
HN
NH
2
NH
2
NH
2
NH
2
O
O
=
,
NH
2
NH
2
NH
2
Figure 3.3 General schemes for synthesis:
(A) symmetrical squaraine,
(B) non-
symmetrical squaraines, and (C) squaraine rotaxanes.
109

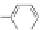




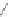
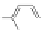




























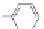


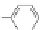







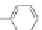



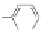






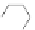












































































































































































































































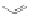








































































Search WWH ::

Custom Search