Biology Reference
In-Depth Information
present very high solvatochromism.
18
We should also mention polymethine
dyes displaying near-IR emission and solvent-dependence of their
lifetime,
48
as well as BODIPY derivatives, capable of switching on-off
their fluorescence in response to solvent polarity changes.
49
Although
these fluorophores have not yet been applied for biomolecular studies,
they appear as attractive building blocks for future high-performance
polarity-sensitive labels of biomolecules.
3. TWO-BAND SOLVATOCHROMIC DYES BASED
ON ESIPT
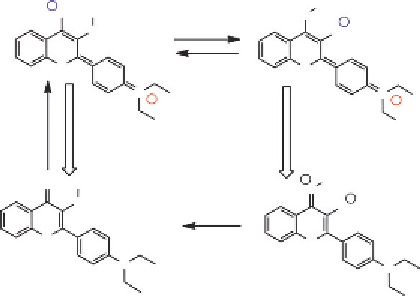
An alternative mechanism of solvent sensitivity can be realized
by utilizing the so-called ESIPT. Particularly interesting ESIPT dyes are
3-hydroxychromones (3HCs), presenting dual emission originating from
the normal excited state (N*) and the ESIPT tautomer (T*) (
Fig. 2.5
).
50
The pathway for ESIPT in 3HCs is provided by the intramolecular H-bond
through a five-membered cycle, which is much weaker than the six-
membered cycle presented by other ESIPT systems. Therefore, it can be
easily perturbed by H-bonding interactions, thus modulating the dual
emission of 3HCs.
-
H
O
H
O
N
*
O
-
T
*
ESIPT
O
O
O
N
N
+
+
h
n
abs
h
n
N
*
h
n
T
*
+
H
O
H
O
O
-
N
T
O
O
O
N
N
Figure 2.5 Photophysical cycle of a 3HC derivative 4
0
-(
N
,
N
-diethylamino)-3-
hydroxyflavone. On electronic excitation (N!N*), a charge transfer from the 4
0
-
dialkylamino group to the 4-carbonyl takes place followed by an ESIPT process
(N*
T transition, the proton remains at the 4-carbonyl group, producing
a zwitterionic T state that rapidly converts into the stable N state.
!
T*). After T*
!



Search WWH ::

Custom Search