Biology Reference
In-Depth Information
the peptide backbone, an amino acid derivative of Prodan, namely,
6-dimethylaminonaphtoyl alanine (Aladan), was synthesized independently
by two research groups.
33,34
The probe allowed monitoring binding of
S-peptide with ribonuclease S
34
and estimation of the local dielectric constant
of the B1 domain of the staphylococcal protein G at different sites.
33
Later,
this amino acid was successfully applied to the study of delta-opioid receptor
antagonist binding.
35
2.3. Improved Prodan analogues
The key weakness of Prodan is its absorption in UV (360 nm), which limits
its cellular applications. In order to shift the absorbance of Prodan to
the red, Lu
et al.
20
synthesized its benzo-analogue, 2-propionyl-6-
dimethylaminoanthracene, Anthradan. This dye showed the desired
red-shifted absorption (around 430 nm), but its brightness was limited
because of its low absorption coefficient. Recently, we extended the elec-
tronic conjugation of Prodan by substituting its naphthalene core with
fluorene.
15
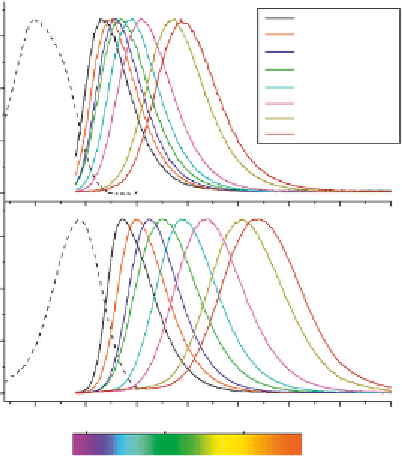
The obtained fluorene derivative, FR0 (
Fig. 2.3
), showed a
Solvent / E
T
(30)
A
Toluene / 33.9
0.9
Dioxane / 36.0
THF / 37.4
EtOAc / 38.1
Acetone / 42.2
0.6
Acetonitrile / 45.6
Ethanol / 51.9
Methanol / 55.4
0.3
0.0
B
0.9
0.6
0.3
0.0
350
400
450 500 550
Wavelength (nm)
600
650
700
Figure 2.4 Absorption (dash) and fluorescence (solid) spectra of Prodan (A) and FR0 (B)
in organic solvents of different polarities. Absorption spectra were recorded in toluene.
E
T
(30)—empirical polarity index.
11
Data from Ref.
15
.

Search WWH ::

Custom Search