Biology Reference
In-Depth Information
S
1
(Franck-Condon state)
S
1
(solvent-relaxed state)
+
-
+
-
Polarity
h
n
h
n¢
S
0
(transient state)
S
0
(ground state)
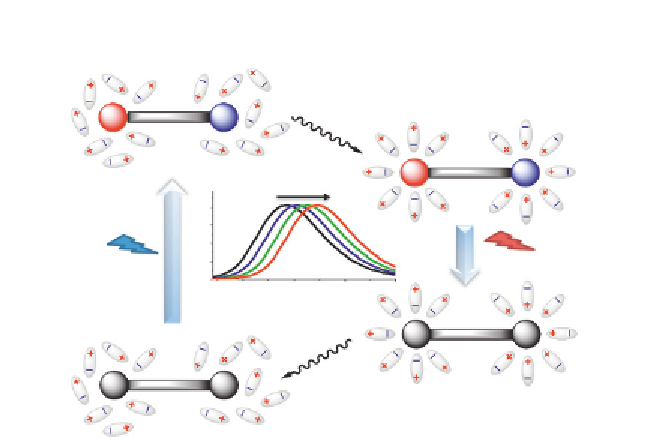
Figure 2.2 Simplified diagram explaining the phenomenon of solvatochromism.
fluorophore through H-bonding and thus can also decrease the energy of
S
sol
1
state. Therefore, both dipole-dipole and H-bonding interactions in
polar solvents can shift the emission of these dyes to the red. Water is a
highly dipolar molecule as well as an exceptionally strong H-bond donor.
Therefore, its effect on the emission color of the solvatochromic dyes is
particularly drastic and can be used for detection of biomolecular
interactions as shown in
Fig. 2.1
. An additional important property of
most environment-sensitive dyes is their poor fluorescence intensity in
water, because of intermolecular electron or proton transfer. Therefore,
incorporation of these dyes into proteins and lipid membranes usually
increases strongly their fluorescence intensity as a result of efficient
screening of these molecules from bulk water.
6,8,13
An unusual class is the
two-band solvatochromic fluorescent dyes based on 3-hydroxychromone
(3HC) derivatives. Because of excited-state intramolecular proton transfer
(ESIPT), they show two emission bands, which change their relative
intensities in response to solvent polarity.
8
This chapter will briefly
present the design and applications of single-band environment-sensitive
dyes, with the main focus on the dyes based on 3HC. For more details
on single-band solvatochromic dyes and their biological applications, the
reader should see the excellent review by Imperiali
et al.
6
Search WWH ::

Custom Search