Biology Reference
In-Depth Information
9. ACRIDINONES
9.1. Overview
Substitution of the oxygen in the phenoxazine core with a quaternary car-
bon to yield 9
H
-dialkylacridinones elicits a further bathochromic shift in
spectral properties. The most widely used acridinone is 7-hydroxy-9
H
-
(1,3-dichloro-9,9-dimethylacridin-2-one) (DDAO;
76
;
Fig. 1.7
), which
displays
10
4
M
1
cm
1.
3,118
DDAO enjoys this utility because of its straightforward synthesis and
because the two chloro substituents ensure a low phenolic p
K
a
value of
5.0.
119
Like the other phenolic dyes, the phenolate is the most
fluorescent form; alkylation or acylation diminishes fluorescence,
providing a means to fluorogenic compounds. One caveat with DDAO
is the relatively high fluorescence of the
O-
alkylated or
O
-acylated form,
although this is mitigated by the dramatic (
>
200 nm) hypsochromic shift
in absorbance when derivatized.
l
max
/
l
em
¼
646/659 nm and
e
¼
5.6
9.2. Fluorogenic enzyme substrates and photoactivatable
probes
Fluorogenic molecules based on DDAO are surprisingly limited, especially
given the red-shifted spectra of the released fluorophore, which minimize
interference from endogenous or exogenous fluorescent molecules.
118
The acridinone scaffold has been used to create a few useful substrates, in-
cluding phosphatase substrate
77
120
and
b
-galactosidase substrate
78
.
118
The
sulfatase substrate
79
has also been reported and found useful to assay aryl
sulfatases, an important enzyme for bacterial infection.
121
Attachment of
Cl
HO
O
N
Cl
76
Enzyme substrates
Photoactivatable fluorophores
OH
HO
NO
2
Cl
Cl
Cl
O
OH
2-
O
O
O
3
PO
O
O
O
HO
Cl
N
N
Cl
N
Cl
80
77
78
Cl
-
O
3
SO
O
O
N
Cl
O
79
3
Figure 1.7 Fluorogenic acridinones.





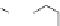












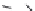














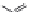


















































Search WWH ::

Custom Search