Biology Reference
In-Depth Information
c
I
0
I
l
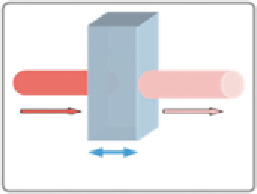
Figure 5.5 Physical parameters implicated in the absorbance measurements. I
0
is the
intensity of the incident light. I correspond to residual intensity after absorption by
the sample. l is the path length of the sample.
2.3. The emission process
When a molecule has been promoted to an excited state upon the absorption
of electromagnetic radiation, it necessarily returns to the ground state through
competition between radiative (
K
r
) and nonradiative (
K
nr
)pathways.Thera-
diative pathways involve photon emission, and nonradiative pathways include
energy transfer through collisions, resonance energy transfer through near-
field dipole-dipole interactions (such as FRET detailed in the next section),
and photochemical decomposition. A change in the vibrational and rotational
states of the molecule can also cause a loss of energy via a nonradiative process.
5
The Jablonski diagram shown in
Fig. 5.4
illustrates the balance of energy
through the excitation-relaxation cycle.
The difference in energy (or wavelength) between the absorbed and the
emitted photons is known as the “Stokes shift” shown in
Fig. 5.6
. This phe-
nomenon was first described by Sir G. G. Stokes in 1852. A large Stokes shift
is often highly desirable for simplifying the wavelength separation between
the fluorescence emission and the excitation.
6
There is competition between the different de-excitation processes pre-
viously discussed (
K
r
and
K
nr
). The quantum yield (F) is the ratio of the
number of photons emitted to the number of photons absorbed. It can also
be described using the rates of radiative (
K
r
) and nonradiative (
K
nr
) processes
of de-excitation.
K
r
K
r
þ
F
¼
½
5
:
4
K
nr
The quantum yield can vary from 0 to 1, where 0 corresponds to non-
fluorescent materials and 1 corresponds to highly fluorescent materials in
which each photon absorbed results in an emitted photon.


Search WWH ::

Custom Search