Biology Reference
In-Depth Information
A
B
360
0.16
hDim1
hDim2
0.14
350
0.12
340
0.1
0.08
330
0.06
0.04
320
0
123456
7
0
1234
GuHCl (M)
567
GuHCl (M)
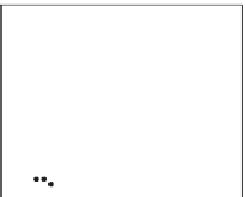
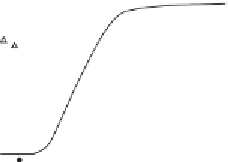
Figure 4.11 Monitoring protein/protein interaction using chaotropic agent. Unfolding
transition of hDim2 was monitored by fluorescence spectroscopy. (A) GuHCl-induced
unfolding transition curves of hDim1 and hDim2 followed by a shift in the fluorescence
emission maximum wavelength of hDim1 (triangle), hDim2 (filled circle). Experiments
were performed at 25
C using a concentration of 3 mM protein. (B) Steady-state anisot-
ropy of hDim2 (closed circle) and hDim1 (triangle) was determined at an emission wave-
length of 340 nM upon excitation at 290 nm in the presence of
increasing
).
68
concentrations of GuHCl (adapted from Simeoni
et al.
range, in contrast to Dim1 homolog, and that Dim2 dimerization regulates its
association with the splicesome machinery.
68
Noncovalently linked external probes can also be applied to monitor
changes in protein/protein interactions. 1-Anilinonaphthalene-8-sulfonate
(ANS) or bis-ANS interacts with hydrophobic pockets that are accessible at
the surface of proteins and has been used to follow transition states upon dis-
sociation and unfolding processes. Moreover, spectral properties of ANS
(emission 490 nm and excitation 340 nm) are compatible with tryptophan
for FRET measurements. ANS and bis-ANS were used to monitor the dis-
sociation of heterodimeric RT. The interface between the two subunits
(p66 and p51) involves large hydrophobic patches and the dissociation of
RT results in a large increase in the fluorescence of the probe due to
noncovalent interactions of ANS to exposed surface hydrophobic motifs
on the subunits, thereby providing a good signal for following RT dissoci-
ation in a time-dependent manner (
Fig. 4.12
).
69
Another interesting alternative to monitor protein/protein interactions is
the use of fluorescently labeled substrates, which, upon binding, reflect the
formation of stable or/and active enzymatic complexes. Themajor advantage
of this approach lies in the ability it provides to discriminate between active
and inactive forms of the protein/enzyme. Two scenarios can be observed:
either the binding site is located and formed by the protein/protein interface;






































































































Search WWH ::

Custom Search