Biology Reference
In-Depth Information
1
300
0.8
250
200
0.6
150
0.4
100
0.2
50
0
0
0
2000
4000
6000
8000
10,000
CADY (nM)
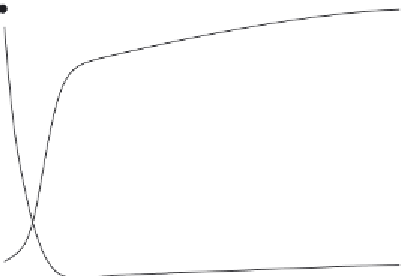
Figure 4.10 Fluorescence analysis of peptide/nucleic acid interactions through the for-
mation of CADY/siRNA complexes. While a strong quenching of the fluorescence inten-
sity of a FITC-labeled siRNA is induced by the presence of CADY peptide (solid label), a
net increase in the fluorescence polarization is also detected (opened label), suggesting
both interactions and formation of a large complex between peptides and the siRNA
(adapted from Deshayes et al.).
57
quenching, enhancement of the quantum yield has also been observed.
54,58
However, one has to keep in mind that the hydrophobic part of the
fluorescent probe confers a hydrophobic anchor for the peptide/cargo
interactions: first through
P
-stacking on the probe, then through
electrostatic interactions between charged residues and the phosphates.
Nevertheless, the extrinsic approach provides alternative information
through fluorescence anisotropy/polarization measurements. Indeed, the
probe possesses a degree of freedom that may vary in the presence of
peptides. The variation of the steric environment of the probe induces a
variation in its degree of freedom, resulting in a modification of
fluorescence polarization. As shown in
Fig. 4.10
, in the case of CADY/
siRNA complexes, a net fluorescence quenching of an FITC (fluorescein
isothiocyanate) labeled siRNA was also associated with a clear increase in
fluorescence polarization.
55-57
These data tend to demonstrate that a
strong reduction in the degree of freedom of the FITC conjugated to the
siRNA occurs in parallel to fluorescence quenching. Thus both
fluorescence intensity and polarization variations support the formation of
mixed peptide/siRNA complexes.










































































































Search WWH ::

Custom Search