Biology Reference
In-Depth Information
O
O
O
HO
O
O
A
Blocking
group
O
O
Esteras
e
O
CO
2
H
O
Nonfluorescent
Fluorescent
2
1
B
O
N
N
B
F
F
O
F
F
-
O
O O
B
N
N
N
+
P
O
O
O
3
Phospholipase
FRET
O
N
N
B
OH
F
F
Nonfluorescent
4
Fluorescent
+
HO
F
F
O
-
O O
B
+
N
N
P
N
O
O
O
5
C
Fluorophore
Formation
HO
OH
HO
OH
HO
O
O
h
u
-EtOH
CO
2
Et
OEt
Nonfluorescent
Fluorescent
6
7
O
D
Hydrophobic
environment
N
O
O
N
O
O
Lipid
N
N
Nonfluorescent
Fluorescent
8
8
-
O
O
O
E
-
O
O
O
F
F
Analyte
2-
F
F
Ca
2+
-
-
O
2
C
N
CO
2
O
O
O
O
O
N
O
Ca
Fluore
scent
Nonfluorescent
N
O
-
-
O
2
C
N
CO
2
O
O
O
9
O
9-Ca
2+
O
Figure 1.1 Modes of fluorescence modulation involving small molecule fluorophores.
eliminates fluorescence. Fluorescence is restored by removal of this group
through an enzyme-catalyzed reaction, photolysis, or another covalent bond
cleavage. A classic example is fluorescein diacetate (
1
) shown in
Fig. 1.1A
.
Acetylation of the phenolic oxygens of fluorescein forces the molecule to
adopt a nonfluorescent, “closed” lactone form. Hydrolysis of the acetate es-
ters by chemical or enzymatic means yields the highly fluorescent fluorescein
in the “open” form (
2
;
10
4
M
1
cm
1
,
l
max
/
l
em
¼
490/514 nm,
e
¼
9.3
0.95)
.
3,4
Control of the open-closed equilibrium in fluoresceins and
rhodamines is a versatile method for constructing fluorogenic biological
probes (see
Sections 6 and 7
).
and
F
¼























































































































































































































































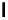

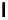


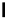

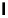

















Search WWH ::

Custom Search