Biomedical Engineering Reference
In-Depth Information
Furthermore, fused enzyme components can be manufac-
tured more efficiently in a single process.
against these key factors is critical for determining the
degree to which a product will be prescribed by physicians,
and therefore the level of sales or profits it generates. In
earlier stages of development, the factors also have a bearing
on the ability to attract licensing deals for product candidates
or technology platforms.
3.2 FACTORS FOR COMMERCIAL SUCCESS
The purpose of this section is to summaries the character-
istics that FPs must possess if they are to stand greater
chances of achieving commercial success. The ability of a
product to generate profits for a biopharmaceutical company
does not depend on the specific protein structure or mecha-
nism of action per se, but instead on how it is positioned to
meet needs of the marketplace against available alternatives.
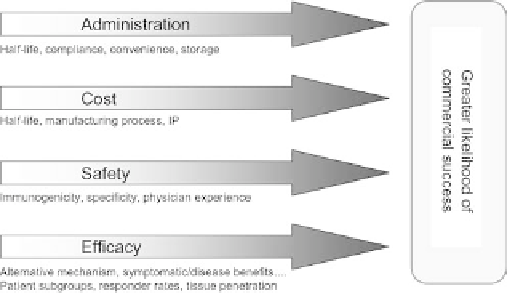
This positioning can be measured on the basis of four key
factors for commercial success: efficacy, safety, administra-
tion, and cost (Figure 3.3).
As discussed earlier in this chapter, there will often be a
range of FP and non-FP approaches that drug developers can
employ to modulate a particular drug target or disease
pathway. Where alternative products are available to physi-
cians in the market, commercial success will be dependent
on some clinical advantage being offered or comparative
cost. Although further factors such as launch timing and
promotional activity also have a key bearing on the com-
mercial success of a product, these are not considered in this
section, since they are independent from the inherent prop-
erties of an FP product. Similarly, there can be wider
academic value in scientific discoveries or patients can be
given products in a charitable manner and the success of a
drug product can also be measured by the improvement to
patient health or cost savings to healthcare providers. How-
ever, this chapter relates only to the point of view of
generating profit as a commercial business.
The development of many FPs has been discontinued in
the past, not because of failing to achieve the intended
mechanism of action as such, but because the performance
against the key factors of commercial success was insuffi-
cient versus the offerings of competing products. Positioning
3.2.1 Administration
Approximately half of the FPs identified are based on half-
life extending technologies to allow practical or less-
frequent dosing. Of these, 25% are specifically positioned
as next-generation versions of existing short half-life thera-
peutic proteins, each of which is either partnered with or
invented by large pharma/biotech companies. Other half-life
extending FPs are developed where a novel protein or
protein fragment would not be practical without the longer
duration of action. However, across the board, drug devel-
opers should seek to ensure that the administration of a
product is sufficiently robust versus competitors. As well as
offering greater convenience, less-frequent dosing can also
aid compliance to the treatment regime, improving patient
outcomes. It should though be noted that these benefits must
be considered in the context of the disorder being addressed
and its severity. There may be only limited tolerance from
payers to accept higher prices for greater convenience/com-
pliance and this is of particular significance where alter-
natives are due to become cheaper in generic or biosimilar
form. Longer half-life products can though require less
physical product to be administered, giving manufacturers
a stronger position in terms of pricing and profitability. This
is likely to have been taken into account by largest global
generics company, Teva, before its February 2008 acquisi-
tion of albumin fusion player CoGenesys.
Earlier in this chapter a wide range of half-life extension
technologies were highlighted; both FP and non-FP. These
typically differ in terms of immunogenicity, level of impact
on partner protein activity, freedom to operate restraints
and manufacturing cost. A further solution to the incon-
venience of frequent dosing is to develop drugs that are less
burdensome to administer. Biologics are typically only
available as injectable formulations since large hydrophilic
peptides are broken down in the gut and do not readily
pass across external surfaces of the body. Furthermore,
generally being more expensive than small molecule drugs,
most biologics are, therefore, only used for comparatively
severe indications.
Only one FP in Phase II development or beyond is
noninjectable. NexBio's Fludase (DAS181) is a powder
formulation administered over 3 days via inhalation for
the treatment of influenza. In this exception, inhalation
represents a practical option since Fludase is designed to
act by binding to the human respiratory tract. However,
various emerging protein technologies including novel FP
FIGURE 3.3
Key factors for commercial success.
Search WWH ::

Custom Search