Biomedical Engineering Reference
In-Depth Information
predicted surface accessible murine-sequence heavy chain
amino acids, one is buried deep in the hydrophobic binding
pocket (GluH42).
The binding cleft of CVX-2000 was characterized func-
tionally by fluorescence spectroscopy and structurally by X-
ray crystallography. The unique reactivity and the apparent
pKa of
e
10.2) in an aqueous environment. At
the standard fusion pH of 6.5, the Henderson-Hasselbach
equation predicts a Lys
-amino group (
-amino with a pKa of 5 to be
150,000-fold more nucleophilic than a typical lysine side
chain. Under those conditions over 99.9% of surface lysines
are protonated, thus nonreactive. Therefore, of the 88 lysines
present in the primary sequence of CVX-2000, the only
lysines expected to be nucleophilic enough to fuse with the
electrophilic linker would be the two LysH93 residues.
e
5-6 for the active site Lys H93 is maintained during
the humanization process. The hydrophobicity of the bind-
ing cleft of CVX-2000 was investigated by the use of the
hydrophobic probe molecule 1-anilinonaphthalene-8-sul-
fonic acid (ANS) [12]. ANS has very little fluorescence
emission in aqueous solutions. However, when associated
with a hydrophobic region of a protein, a substantial blue
shift and increase in fluorescence with emission maximum
around 460 nm is observed. When CVX-2000 is incubated
alone in solution with ANS, a strong fluorescent emission is
observed. The emission signal is significantly reduced and
red-shifted after CVX-2000 has been conjugated to a dike-
tone, presumably by displacement of ANS from the hydro-
phobic binding cleft. The resulting fluorescent emission
spectrum of conjugated CVX-2000 is comparable to the
control sample of polyclonal IgG incubated with ANS, as
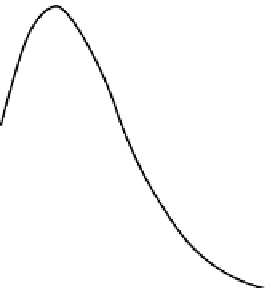
seen in Figure 38.4A. The apparent pKa of LysH93 of CVX-
2000 was determined by measuring the binding activity of a
model diketone substrate (2,4-pentanedione) as function of
pH. The binding of 2,4-pentanedione was followed by
measuring the UV absorbance signal at 316 nm that results
from the vinylogous amide formed from the reaction of the
2,4-pentanedione with LysH93 in the active site of CVX-
2000. Figure 38.4B shows the titration experiment of CVX-
2000 with 2,4-pentanedione. This figure suggests an appar-
ent pKa of approximately five for the two reactive LysH93s
of CVX-2000 under equilibrium reaction conditions. This is
substantially lower than the typical value for the lysine
38.2.2 Linker Design
The early examples of CovX-Bodies targeting integrin,
endothelin and CCR5 receptors utilized linkers based on
the 1,3-diketone hapten used to raise 38C2. The advantage
of this linker system was that the fusion reaction was, for all
practical purposes, instantaneous and the fusion product
resulted in the formation of a vinylogous amide in the active
site that could be detected spectroscopically, which made it
possible to easily follow the course of the fusion reaction and
characterize the fusion products. However, a major concern
with diketone linkers was that the fusion reactions between
the antibody and the targeting-pharmacophore were medi-
ated via a reversible, albeit, stable bond. The reversibility of
the bond presented several challenges for the development
of CovX-Bodies. For example, the strength of the interaction
between the linker and the antibody, and thereby the degree
of reversibility was expected to be influenced by the nature
of the linker and the pharmacophore. This, in turn, intro-
duced an added variable that needed to be studied and
optimized for each class of fusion product. Additionally,
the in vitro bioactivity assessment of CovX-Bodies was
complicated by the fact that the observed activity could
be a result of the payload released from the antibody rather
(A)
(B)
250000
Catalytic antibody
0.18
0.16
0.14
0.12
0.1
0.08
0.06
0.04
0.02
0
CovX-body
200000
Control lgG
150000
100000
50000
0
400
450 500
Emission wavelength (nm)
550
600
0
2
4
6
8
10
pH
FIGURE 38.4 (A) Hydrophobicity of CVX-2000 binding cleft as determined by ANS probe. (B)
Binding activity of CVX-2000 with pentanedione as a function of pH.









































Search WWH ::

Custom Search