Biomedical Engineering Reference
In-Depth Information
epitopes on a single target protein. By controlling the orien-
tation and flexibility of the binding arms and careful selection
of the target epitopes it may be possible to create high avidity
protein clamps similar to the tandem scFv-based chelating
recombinant antibodies (CRAbs) CRAbs have been demon-
strated to achieve sub-nanomolar avidities through coopera-
tive binding [41]. ACRAb-like biparatopicmolecule based on
the heterodimerizing SEED platform would be expected to
having the avidity advantage of the CRAb together with the
effector mechanisms and pharmacokinetic properties of
normal antibodies. Alternatively, a biparatopic antibody
oriented to favor interaction with different epitopes on
neighboring cell-surface proteins has the potential to form
a lattice of interconnected target proteins. Interestingly,
such a phenomenon was observed using different conven-
tional antibodies that bound distinct epitopes of EGF
receptor [42]. In this case, a combination of two antibodies
that bound to nonoverlapping epitopes of EGFR led to
strong synergistic activation of complement-dependent
target cell lysis. These results suggested that the lattice
clustering induced by the antibodies was, at least in part,
responsible for increased tumor cell killing. Although not
formally demonstrated, it would be anticipated that a
single bispecific antibody that bound to these same epit-
opes would likewise induce EGF receptor clustering and
possibly increased cell lysis.
Other potential opportunities for SEED-based molecules
include the enhancement of biologically active ligands, such
as interferons and interleukins, through improved pharma-
cokinetics and ease of manufacture. Toward this end, a
SEED-based molecule having a single IL-2 moiety fused
to one chain of the molecule has been described that has
prolonged pharmacokinetics when tested in mice [19]. Such
an approach may have advantages in cases where two copies
of an active ligand fused to a single molecule can interfere
with protein production. Alternatively, the SEED platform
may be amenable to generating cytokine traps created by
fusing a pair of different receptor chains capable of creating
a high affinity receptor complex to the two heavy chain
arms. This approach may be less complex than approaches
such as VEGF-Trap where multiple copies of receptor
chains are fused to a homodimeric Fc scaffold [43].
37.4 FUTURE PERSPECTIVES
While preclinical activity has demonstrated the potential
utility of the SEED platform, as with all engineered proteins,
significant challenges can be expected in the future. For
example, even when the interactive forces strongly favor
heavy chain heterodimerization versus homodimerization,
significant variation in expression rates of the different
heavy chain genes may impact the final molecule. While
gene dose can be more readily regulated in transiently
transfected cells, the relative chain expression in stable
transfection may be more difficult to control because of
integration site, copy number, and translation efficiency
variability. From a manufacturability stand-point, produc-
tion complexities often increase in step with the number of
different protein chains comprised in the product. Hence,
molecules such as MetMab that include three or more
distinct chains could be expected to have more challenges
in production than a conventional antibody with two chains.
As found in other bispecific antibody platforms, nonFab
binders often remain a weak link in the overall molecular
stability. Significant effort is required to assess the bio-
physical attributes, such as melting temperature, of the
binder arm components to select the ideal binders or
improve suboptimal ones through stability engineering [44].
In principle, a single bispecific antibody that has two
different specificities could have cost advantages over two
conventional antibodies. However, as many companies have
well established and economically optimized antibody man-
ufacturing platforms, any additional costs seen with a bis-
pecific antibody, whether due to expression or purification
differences, may eliminate any potential cost advantage.
Finally, as with all nonnative proteins, there remains a
risk of immunogenicity for engineered antibodies. Unlike
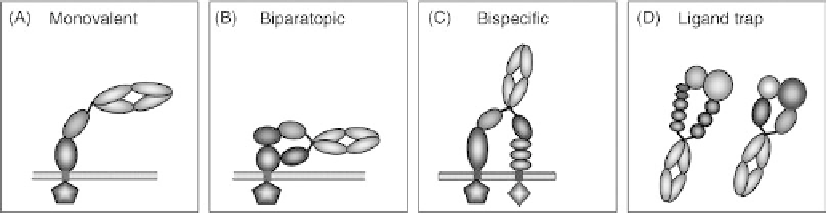
FIGURE 37.6
Potential applications for a heterodimeric Fc-based biotherapeutics platform.
Applications include monovalent binding to reduce risk of agonism or receptor down-regulation
(A), binding to two epitopes on same target (B), binding two distinct targets (C), and as a high affinity
cytokine trap, as either a bispecific antibody or receptor chain fusion molecule (D).
Search WWH ::

Custom Search