Biomedical Engineering Reference
In-Depth Information
produce bivalent and monovalent scFv-SEED and VHH-
SEED proteins. Bispecific SEED-based proteins have also
been produced, for example by fusing one SEED chain to a
Fab's VH-CH domains and the other SEED chain to a scFv,
or by fusing different scFv's to each of the SEED chains
(Figure 37.3D). We have found that the SEED scaffold is
compatible with fusion in different combinations to different
types of binder domains, including Fab, scFv, and VHH
domains, and that these domains still bind their targets.
analyses of SEED-based proteins. In our assays, we com-
pared SEED- and Fc-based proteins to determine if SEED
retains physical properties that are compatible with therapeu-
tic use. For example Fc-based proteins are stable and tolerate
a range of pH conditions; therefore changes in the pH
environment could reveal if SEED-based proteins are signifi-
cantly less stable than Fc-based proteins. Stability specifically
in low pH is also a desirable therapeutic antibody property
because of the low-pH viral inactivation steps required during
production. Here, we show an example of Fc and SEED
scaffold proteins exposed to different pH buffers for 30min,
after which the amount remaining of nonaggregated protein
of the correct size was measured with analytical SEC and the
area under the curve (AUC) of the SEC profile was plotted for
each condition. SEED scaffold showed a similar stability to
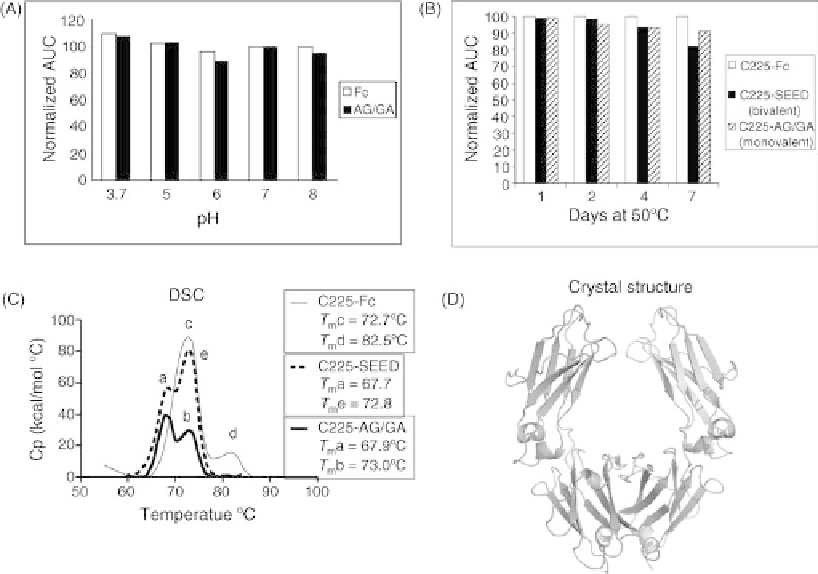
Fc in this range of pH conditions (Figure 37.4A).
37.2.3 Biophysical Properties
To assess the stability and biophysical properties of SEED
proteins, we have performed a variety of assays with the
SEED scaffold and also with the SEED-fusion proteins.
Figure 37.4 shows examples of these types of physical
FIGURE 37.4
Physical testing of SEED molecules. (A) Stability at a different pH values. Example
comparing Fc and SEED scaffold (AG/GA heterodimer) after being held at different pH values for
30min. Amount of protein remaining after 30min was measured by analytical SEC and the area
under the curve (AUC) of the main protein peak was normalized to protein after control incubation.
(B) Stability under temperature stress. Example compares stability of C225-Fc, C225-SEED
(bivalent), and C225-AG/GA (monovalent) after proteins were held at 50
C for 1-7 days. Amount
of protein remaining was measured by analytical SEC and AUC of main protein peak was normalized
to control sample. (C) Differential scanning calorimetery (DSC) analysis. Example compares DSC
profiles of C225-Fc, C225-SEED (bivalent), and C225-AG/GA (monovalent). T
m
values shown are
derived from the midpoint of the indicated peaks. Peaks: (A) CH2
þ
SEED domains; (B) single
C225 Fab domain; (C) CH2
þ
two C225 Fab domains; (D) CH3 domains; and (E) two C225 Fab
domains. (D) Physical analysis by crystal structure determination. Crystal structure of SEED scaffold
(AG/GA heterodimer) was solved to 2.5 A
to determine the SEED design domain folding and
interactions between the domains. (Source: Figure 37.4C reproduced from Reference [37] by
permission of Oxford University Press.)
Search WWH ::

Custom Search