Biomedical Engineering Reference
In-Depth Information
(AG/GA heterodimer) were comparable (
80-100
m
g/mL)
(Figure 37.2B). Analytical SEC profiles comparing Fc and
SEED scaffold (AG/GA heterodimer) show that both types
of proteins were homogenous and of the expected size
(Figure 37.2C). Our methods to determine if the SEED
protein is an AG/GA heterodimer are also illustrated in
Figure 37.2. Owing to their different amino acid composi-
tion, the AG and GA SEED chains have different pI values
and thus a protein that is a heterodimer of AG and GA chains
focuses to a distinct position in IEF gel analysis (theoretical pI
values: AG
with only minor fractions of higher weight aggregated mate-
rial that can easily be removed with preparative SEC as
needed. In contrast to Fc-based proteins, SEED-based pro-
teins are produced by co-expression of two different protein
chains, which in transient transfection is simply achieved by
co-transfection of an AG and GA expression plasmid. Since
the IEF gel and HPLC methods allow measurement of the
percent heterodimer content of the proteins produced, we
determined that with transfection of our standard optimal ratio
of AG and GA plasmids an essentially pure AG/GA hetero-
dimer protein is produced from cells.
7.5).
The example in Figure 37.2D shows that the SEED hetero-
dimer is clearly distinguished from Fc or a GA/GA homo-
dimer. The elution profile of the SEEDAG/GAheterodimer in
reverse phase HPLC is also distinct form the elution of either
the AG or GA SEED chains; the example in Figure 37.2E
shows a nonreduced sample of SEED scaffold (AG/GA
heterodimer) eluting at 11.8min, which is clearly distinct
from the individual AG and GA peaks (eluting at 7.7 and
13.9min, respectively) seen when a sample of the SEED
scaffold (AG/GA heterodimer) was first reduced with dithio-
threitol into its separate chains.
Together, this workflow allowed us to determine that with
transient transfection Fc- and SEED-based proteins are
produced from cultured cells with a similar yield and
¼
8.2; GA
¼
6.4; AG/GA
¼
7.3; and Fc
¼
37.2.2 Therapeutic Formats
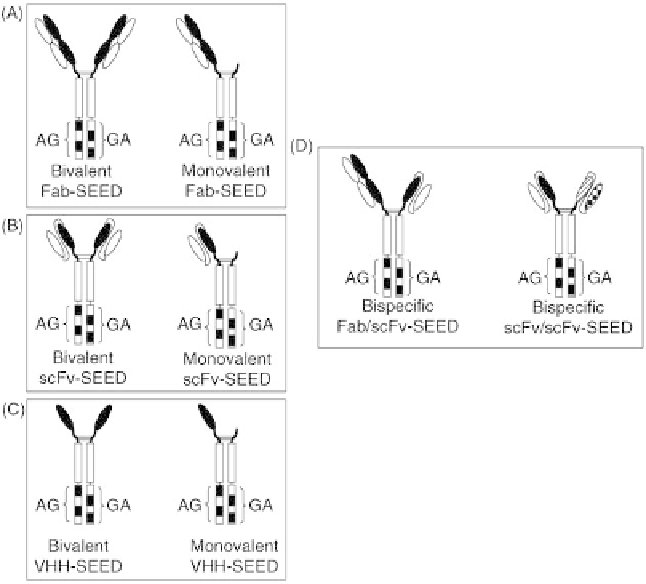
To explore the use of the SEED heterodimer scaffold, we
have produced a number of SEED-based proteins in different
therapeutic formats (Figure 37.3). For example, SEED can be
used with Fab domains to produce bivalent or monovalent
Fab-SEED molecules (Figure 37.3A). This is accomplished
by fusion of one or both SEED chains to the variable heavy
chain (VH) and constant heavy chain (CH) domains of a Fab
and co-expressing the SEED chains with the Fab light chain.
SEED is also compatible with other binder domains; for
example, we have fused scFv domains (Figure 37.3B) or
VHH domains (Figure 37.3C) to one or both SEED chains to
FIGURE 37.3
Versatile therapeutic formats of SEED proteins. SEED has been found to be
compatible with different binder domains and in different combinations. Some examples: (A) Bivalent
and monovalent Fab-SEED proteins. (B) Bivalent and monovalent ScFv-SEED proteins. (C) Bivalent
and monovalent VHH-SEED proteins. (D) Bispecific Fab/ScFv-SEED and ScFV1/ScFV2-SEED.
Search WWH ::

Custom Search