Biomedical Engineering Reference
In-Depth Information
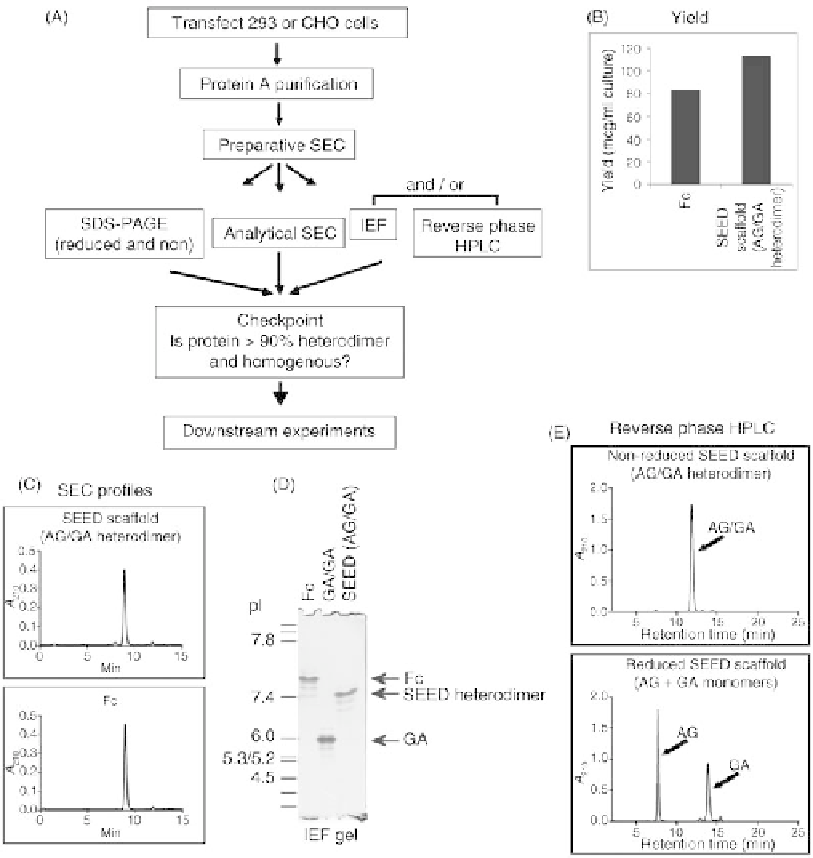
FIGURE 37.2
Expression, purification, and characterization of SEED proteins. (A) Standardized
workflow to produce SEED proteins by transient transfection, purify from medium, and determine if
the purified proteins pass the checkpoint for downstream experiments. SEC, size-exclusion chro-
matography; IEF, isoelectric focusing. (B) Example comparing yield after protein A purification of
IgG1 Fc and SEED. Yield measured as microgram protein A-purified protein per milliliter cell culture
medium. (C) Analytical SEC example comparing Fc and SEED scaffold proteins obtained after single-
step protein A purification. (D) IEF gel analysis example showing distinct focusing of Fc, SEED
heterodimer (AG/GA), and GA/GA homodimer. (E) Reverse phase HPLC example showing different
elution times of SEED scaffold (AG/GA heterodimer) compared to individual AG and GA chains.
molecular weight and percent aggregate of the purified
material was determined by SDS-PAGE (reduced and non-
reduced) and analytical SEC. Since SEED proteins should
be produced as heterodimers of the AG and GA SEED
chains, which have the same size, additional methods
were developed to measure AG/GA heterodimer proteins
versus homodimer and individual chains, specifically iso-
electric focusing (IEF) gel analysis and reverse phase HPLC
analysis. The workflow is designed so after
characterizations there is a checkpoint that requires that
the SEED proteins must be
90% heterodimer and homog-
enous before they can be used in further biophysical and
biological experiments. Proteins were routinely obtained as
>
>
95% homogenous AG/GA heterodimers.
In Figure 37.2, representative data sets are shown to
illustrate this workflow. For example, Fc- and SEED-based
proteins are produced with similar yields (
20-100
m
g/mL),
and in this example the yields of both Fc and SEED scaffold
these
Search WWH ::

Custom Search