Biomedical Engineering Reference
In-Depth Information
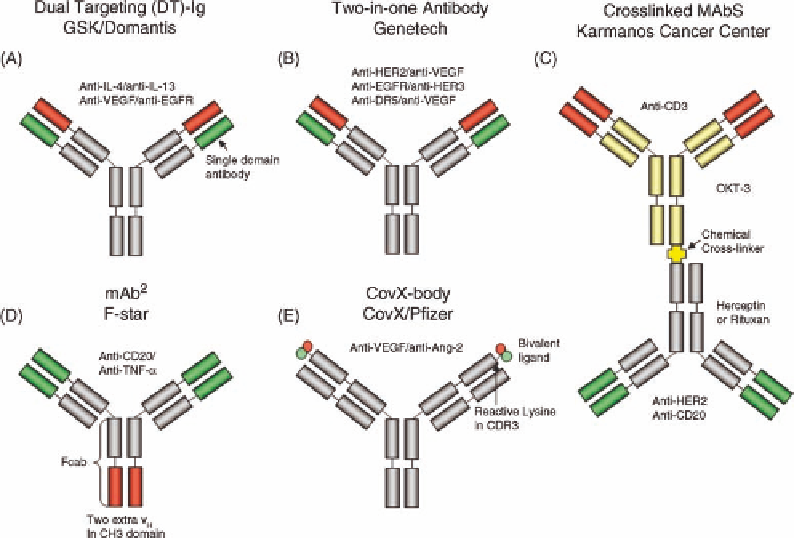
FIGURE 35.2
Symmetric bispecific antibody formats. Red and green colors highlight variable
antibody domains of distinct specificity. Disclosed binding specificities and particular structural
features are labeled.
On the basis of the observation that binding of the mAb
trastuzumab (Herceptin
1
) to the HER2 antigen is primarily
mediated by the three CDRs of the heavy chain variable
domain, researchers at Genentech engineered the CDRs of
the light chain variable domain to recognize a second
antigen using phage display technology [27]. This approach
has been successful and led to antibodies that in their
variable heavy and light chain domains are bispecific for
HER2 and VEGF, EGFR and HER3, or DR5 and VEGF,
respectively [28]. Genentech's “two-in-one” antibodies are
very similar in structure to GSK's DT IgG using single-
domain antibodies (compare Figure 35.2A and B).
A rather simplistic approach to bispecific antibody gen-
eration has been taken by Lum and Davol (Figure 35.2C)
[29]. Commercial mAb OKT3 (Orthoclone
1
) is chemically
cross-linked at a 1:1 ratio with other commercial antibodies,
such as trastuzmab (Herceptin
1
) or rituximab (Rituxan
1
).
The resulting antibody conjugates are used for connecting
cytotoxic CD3-expressing T cells with breast cancer cells
overexpressing HER2 or lymphoma cells expressing CD20.
In preclinical studies, this approach showed robust T-cell
activation and redirected lysis [30]. In clinical practice, T
cells isolated from patients are “armed” ex vivo with the
bispecific antibody conjugate followed by their infusion, a
procedure eventually avoiding systemic cytokine release
reactions otherwise induced by the OKT-3 moiety of the
conjugates. A Phase I study in 19 metastatic breast cancer
patients with an OKT-3/trastuzumab conjugate has resulted
in one partial response and 10 disease stabilizations [31].
The Biotech Company F-star has developed another
solution for introduction of a second binding specificity
into monoclonal IgG1 antibodies by engineering a second
binding specificity into the C-terminal CH3 domain of the
heavy chains (Figure 35.2D). The CH3 domain has loop
structures that allow introduction of CDR diversity analo-
gous to V
H
and V
L
domains by using phage display tech-
nology. The resulting bispecific antibodies with an
additional binding specificity in the CH3 domains are
referred to as “mAb
2
antibodies,” and the isolated Fc
g
part is called an “Fcab.” The CH3 domain of mAb
2
anti-
bodies and Fcabs essentially contains two identical single-
domain antibodies that will bind their antigen by using only
two CDR-like loops. The company has several preclinical
programs. One is a bispecific anti-CD20/anti-TNF-
a
anti-
body in preclinical stage for treatment of rheumatoid arthri-
tis [32]. F-star will collaborate with Boehringer Ingelheim
on the development of up to seven mAb
2
or Fcab antibodies.
A unique approach to bispecific antibodies has been
developed at the Scripps Research Institute and commer-
cialized by CovX (now Pfizer). It is based on a catalytic
antibody with a reactive lysine residue in its binding site.
Dual binding specificity can be introduced to the antibody by
covalently linking small bispecific molecules to the lysine
residues [33]. An antiangiogenic bispecific “CovX-body”
Search WWH ::

Custom Search