Biomedical Engineering Reference
In-Depth Information
[11]. Natural bispecific (polyreactive) antibodies have been
reported to have superior protective activity against human
immunodeficiency virus (HIV) [12].
For this review, we have grouped 37 bispecific antibody
formats into seven distinct classes and present examples of
each class in a separate figure. For each antibody construct,
the bispecifically binding immunoglobulin (Ig) domains are
shown in green and red colors. Particular structural features
are highlighted and labeled, and all constructs are drawn at the
same scale such that their relative sizes can be directly
compared. The development stages of featured antibody
development candidates represent a snapshot at the end of
2010. On the way forward, some candidates will disappear
while others may newly appear in pipeline charts of compa-
nies. In the following sections, seven classes are described and
their opportunities, specific issues, and challenges discussed.
mixture of up to 10 different species of which only one
corresponds to the desired bispecific antibody. Seven species
will have mispaired heavy and light chains and two corre-
spond to the initial mAbs. This situation requires an exten-
sive purification effort and can result in low yields of the
correct bispecific antibody species, which is why researchers
tried to find ways of selectively pairing the two different
heavy chains.
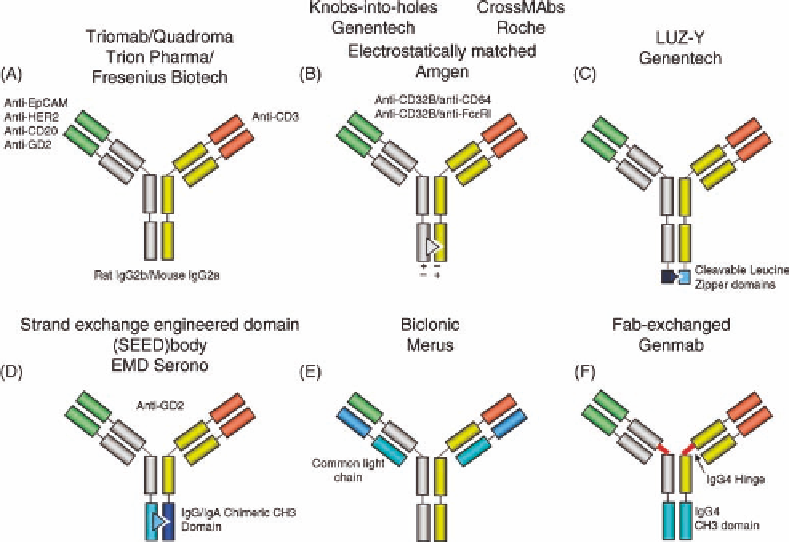
One early solution resulted from the fusion of murine
and rat hybridoma lines [2]. The preferential species-
restricted heavy as well as light chain pairing of rat
IgG2b/mouse IgG2a bispecific antibody species is leading
to the correct bispecific antibody species with high yield
(Figure 35.1A). The German Biotech Company Trion
Pharma in collaboration with Fresenius Biotech has estab-
lished a pipeline of bispecific trifunctional antibodies for
cancer therapy based on their “Triomab” format. These
antibodiesaredesignedtobindwithonearmtocancer
cells expressing frequent tumor-associated antigens—such
as EpCAM, HER2, CD20, or GD2—with their second arm
to the invariant CD3 epsilon signaling chain of the T-cell
receptor (TCR) on T cells, and with their “third arm,” that
is, the Fc
g
part of rat IgG2b and mouse IgG2a, to antigen-
presenting cells. It is believed that this trifunctional bind-
ing between cancer, T, and antigen-presenting cells
35.2 ASYMMETRIC IgG-LIKE BISPECIFIC
ANTIBODIES
The first bispecific antibody format is shown in Figure 35.1,
where the two halves of antibodies are different, or asym-
metric. If two distinct hybridoma lines are fused, the anti-
bodies secreted by the resulting quadroma cells will be a
FIGURE 35.1
Asymmetric bispecific antibody formats. Red and green colors highlight variable
antibody domains of distinct specificity. Gray and yellow label properly heterodimerized heavy and
light chains derived from two different mAbs. Disclosed binding specificities and particular structural
features are labeled. Figure 35.1b depicts three closely related but different formats.
Search WWH ::

Custom Search