Biomedical Engineering Reference
In-Depth Information
FIGURE 34.2
Schematic examples of DARPin fusion proteins. DARPins are represented as gray
sickles. (A) Bispecific DARPins targeting the
a
chain of the membrane-bound high-affinity receptor
for human immunoglobulin E (Fc
e
RI
a
) in two different binding sites (Eggel et al. [40]).
(B) Multidomain construct binding the knob of adenovirus as well as Her2, generated to retarget
adenovirus to Her2-expressing cells. The knob is represented as white circle (Dreier et al. [44]).
(C) DARPin-ETA fusion protein for targeted tumor therapy harnessing the cytotoxic activity of ETA.
ETA is represented as dark gray oval
(Martin-Killias et al. [47]).
maturation. We were thus curious to see, what would happen
if one connected two high-affinity DARPins (90 pM affinity
to the ErbB2 receptor [31]) in a row. Indeed, the construct,
which again showed unchanged biophysical properties com-
pared to the single-domain DARPin, exhibited an apparent
quasi-covalent affinity [30]. The same DARPin could also
easily be combined with other DARPins with no change of
production properties. For example G3 was combined with a
nonbinding DARPin. In this construct G3 had the same
properties as single-domain G3, meaning that the fusion
partner did not influence the activity of G3 [30].
Bispecific or bivalent approaches have also been published
for protein Z, fibronectin, and lipocalin (see Table 34.1). Two
lipocalins were fused head-to-tail resulting in a construct that
could bind fluorescein and digoxigenin at the same time [41].
Similarly, bivalent protein Z variants binding Her2 or bispe-
cific proteinZ variants bindingHer2 and EGFRwere reported
[42]. Recently, a fibronectin-derivative construct binding
bispecifically to EGFR and IGF-1R has been reported to
act as potent anticancer therapeutic [43]. Such molecules
clearly can bring targeted therapy to a next level. It will be
interesting to see what will happen with such molecules in a
clinical context. Efficacy is likely to improve, yet, toxicity
might like-wise also increase in parallel. With nonantibody
binding proteins, it is easy to generate many combinations
and rapidly achieve product candidates that can go to clinical
testing, either validating or invalidating a certain targeting
combination.
34.3.2.2 Multispecific Constructs Going beyond two-
domain fusions is certainly more interesting. This is easily
feasible with DARPins and was exemplified recently by a
multidomain DARPin consisting of four repeat domains
used to re-target adenovirus [44]. Binding DARPins against
the knob of the virus were generated and then different
valencies were assessed. A stoichiometry of 1 DARPin per
knob subunit, corresponding to a trivalent DARPin, was
found to be ideal, attaching the DARPin construct tightly to
the virus and forming a stable complex. This trivalent
DARPin was linked to a fourth DARPin targeting ErbB2.
Importantly, the quadruple DARPin construct was stable on
the virus and could efficiently be used to specifically guide
TABLE 34.1 Multispecific and Multidomain Molecules Involving Nonantibody Binding Proteins
Target(s)
Scaffold Involved
Number of Domains
Particularities
Reference
Her2
DARPin
2
Quasi-covalent binding by bivalency
[30]
Fc
RI
a
DARPin
2
Biparatopic receptor antagonist
[40]
e
Ad-fiber knob and Her2
DARPin
4
Trivalent knob binder and Her2 targeting
[44]
Her2
Affibody
2
Bivalent
[48]
EGFR
Affibody
2
Bivalent
[49]
Her2 and EGFR
Affibody
2
Bispecific
[42]
Her2 and HSA
Affibody
2
Half-life extended Her2 binder
[50]
TNF-
a
Affibody
2-3
Bispecific or trispecific TNF inhibitors
[51]
Fluorescein and digoxigenin
Lipocalin
2
Bivalent
[41]
EGFR and IGF-1R
Fibronectin
2
Bivalent
[43]
IL-6 and IgG
Avimer
4
Half-life extended (IgG-binding)
trivalent IL-6 antagonist
[52]



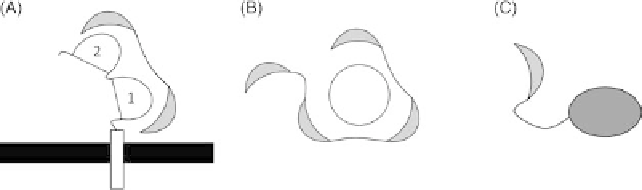


Search WWH ::

Custom Search