Biomedical Engineering Reference
In-Depth Information
62.5
60
57.5
55
52.5
50
47.5
45
FIGURE 33.6
Effect of different thrombolytic on %TIMI 3 in similar Phase II angiographic studies.
No significa nt difference s betwee n the treat ment
groups were observed for the secondary effica cy
parame ters: corr ected TIMI fra me count 60 min after
amedip lase administrat ion; reso lution of ST elevations
at 180 min (3 h postdose) ; 30-da y mor tality; inciden ce
of in-hospi tal major nonfatal cardiovascular events.
The safe ty profiles of the body weight-a djusted doses
of 1.0 and 1.2 mg/kg ame diplase observed du ring this
study d id not
Amedipl ase is endowed wi th a remar kable thromb olytic
efficacy, has a relat ively long-last ing plasmatic kinetic
allowing a single bolus administ ration, is devoid of immu -
nogenic propert ies in huma ns and may have a potential ly
lower inciden ce and severity of minor and major blee ding.
Amedipl ase shoul d therefore be consi dered as ele ctive drugs
for early thr ombolys is (even in a preho spital set ting) with
dosing tailored to each sing le patient, and hence with a
reduced risk of severe blee ding as side effects. The need of
this kind of drug is still high irresp ective of the large use of
mechani cal methods (angioplast ic, stent) for rapi d coron ary
reperfu sion due to the fact that the hospi talization is not
available everywhere, particula rly in not West ern countri es.
Other potential indicati ons are pulmona ry emb olism,
deep venous thrombosis, ischemic stroke, and maintenance
of patency of extracorporeal shunts where thrombolytics
have been found active.
reveal any
remar kable difference s
between the two dose groups .
33.5 HISTO RICAL COMPAR ISON WIT H OTH ER
THROMB OLYT ICS
Amedipl ase was compare d to the reteplase , tene cteplase,
and alteplase , for % TIMI 3 in similar angiog raphic trials
[25,26,28,29 ]. As shown in Figur e 33.6, at 60 min from
administrati on of thromb olytic, it had the best performanc e
on this parame ter. This comparison has some obvious
limitation because o f the different condi tions used in the
trials and further and more complete studies shal l neces sary
to better analyze the difference s found.
ACKNOWLEDGMENT
The authors would like to thank Mrs. Elena Oberto for her
secretarial assistance.
REFERENCES
33.6 CON CLUSIO NS AND FUTURE
PERSPEC TIVES
1. “WHO Disease and injury country estimates”. World
Health Organization. (2009) Available at http://www.who
.int/healthinfo/global_bur den_disease/estimates_count ry/en/
i n de x . h t m l . R e t r i eved 2009 November 11.
2. Xu J, Kochanek, Murphy SL, Tejada-Vera B. (2010) Deaths:
final data for 2007. Nation. Vital Statist. Rep. 58, 1-135.
3. Van de Werf F, Bax J, Betriu A, Bolmstrom-Lundqvist C, Crea
F, Falk V, et al. (2008) Management of acute myocardial
infarction: in patients with persistent ST-segment elevation.
Eur. Heart J. 29, 2909-2945.
4. Menon V, Harrington RA, Hochman JS, Cannon CP, Goodman
SD, Wilcox RG, et al. (2004) Thrombolysis and adjunctive
therapy in acute myocardial infarction. Chest 126, 549S-575S.
Amedipl ase is the outcome of a project aimed to iden tify and
develop a newer thr ombolytic agent char acterize d by poten t
fibrinoly tic activity, enhanc ed select ivity for the thrombus
(fibrin-spec ificity ), prolonged plasma half-l ife, absen ce of
immunogeni city, and also a low manufactur ing cost. In this
regard, amedip lase is consider ed a third-gene ration thr om-
bolytic with advantages over the previous ones such as
streptoki nase, urokinas e, and alteplase in terms of effica cy
and handling .
Amedi plase has confirmed in preclinic al and clini cal
studies a promis ing pharmac odynami c and safe ty profile.

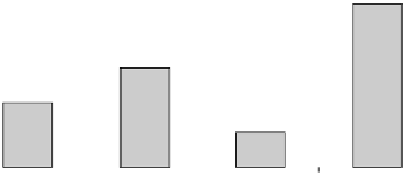










Search WWH ::

Custom Search