Biomedical Engineering Reference
In-Depth Information
33.3 PRECLINICAL STUDIES
concentrations (
2
m
g/mL). In this respect, the hybrid
is well comparable to t-PA [10].
33.3.1
In Vitro
Biological Properties
33.3.1.4 Inhibition by Plasma Protease Inhibitors Like
scu-PA, the hybrid is insensitive to inhibition. Indeed, incu-
bation in plasma for 24 h at 37
C, at a concentration of
500 ng/mL, is not associated with a noticeable inhibition of
the activity (
The recombinant hybrid retains some important properties of
the parent molecules t-PA and scu-PA. Like t-PA, it requires
fibrin to fully express PAactivity thus granting a fibrin-specific
thrombolytic activity. Like scu-PA, it behaves as a zymogen,
being insensitive to plasma protease inhibitors, including the
fast acting PAI-1, and requiring conversion to an active (two-
chain) formfor full expression of enzymatic activity. The latter
property may further increase the fibrin specificity by limiting
the activation of the molecule to the thrombus surface where
plasmin is generated and protected from inhibition [10].
10%). t-PA, under the same experimental
conditions, is effectively inhibited, with a t
1/2
of about
90min. Resistance to inhibition was also demonstrated using
purified recombinant type 1 plasminogen activator inhibitor
(rPAI-1) [10]. Amediplase has also been shown to be more
resistant than t-PA [11] or scu-PA [12] to the antifibrinolytic
activity of TAFI (thrombin activable fibrinolysis inhibitor). At
therapeutic concentrations both amediplase and tenecteplase
are marginally affected by TAFI [12].
<
33.3.1.1 Plasminogen Activator Activity The specific
activity of amediplase, measured by amidolytic assay and
fibrin plate is 2.1
10
6
t-PA equivalent units
per milligram, respectively. On a molar basis, the hybrid is
twofold more active than t-PA. PA activity of amediplase is
reduced by more than 98% in the absence of CNBr-digested
fibrinogen, indicating that, like t-PA, the hybrid requires
fibrin for the expression of its activity [10].
10
6
and 2.5
33.3.1.5 Binding to Fibrin When the binding to forming
fibrin was studied, a 40% binding of the hybrid was obtained
at a starting fibrinogen concentration
3.2mg/mL. This
figure is significantly lower than that obtained with t-PA,
which is almost totally (
>
80%) found in association with
fibrin at a fibrinogen concentration as low as 0.2mg/mL [10].
33.3.1.2 In Vitro Thrombolytic Activity This property
was studied using a
125
I-labeled (human) blood clot
(125
m
L) immersed in autologous plasma (1 mL). Amedi-
plase, at a concentration of 500 ng/mL, induces clot lysis at a
rate comparable to that of a similar concentration of t-PA
whereas, at lower concentrations, it is less active than the
reference enzyme. Dose-response curves indicate that the
extent of lysis by amediplase is not linear with the enzyme
concentration. This behavior resembles that of scu-PA [10].
33.3.1.6 Clot Penetration Amediplase binding to fibrin
clot is specific being fully inhibited by
-aminocaproic.
Plasma clot penetration was studied during pressure-driven
fluid permeation. Amediplase, at variance to alteplase, was
able to enter the clot without significant hindrance. This
observation was also confirmed in diffusion-driven clot
penetration study using confocal microscopy. Alteplase
was detected on or close to the clot surface, while amedi-
plase was also detected deep inside the clot [13].
In a further set of experiments (Figure 33.3), it has been
shown that, independently from the size of the human clot,
amediplase possesses, at therapeutic concentrations, a faster
lysis time than tenececteplase or, evenmore, than scu-PA [12].
e
33.3.1.3 Fibrin Specificity In plasma, in the absence of
fibrin, amediplase induces little activation of fibrinolysis
as indicated by the fact that significant plasminogen and
a
2-antiplasmin consumption is observed only at high
45
40
35
30
25
20
15
10
5
0
TNK (2.5
g/mL)
Ame (5.0
µ
g/mL)
µ
Big clot
(1 mL)
Medium clot
(0.2 mL)
Small clot
(0.04 mL)
FIGURE 33.3
Comparison of in vitro lytic effect of amediplase (Ame) and tenecteplase (TNK)
with clots of different size. See Reference [12].
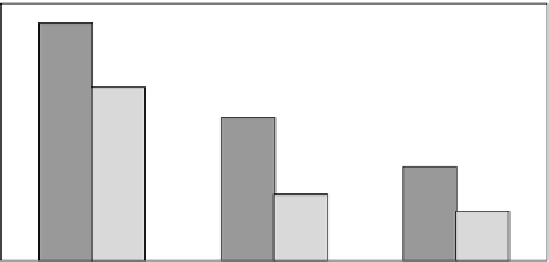











Search WWH ::

Custom Search