Biomedical Engineering Reference
In-Depth Information
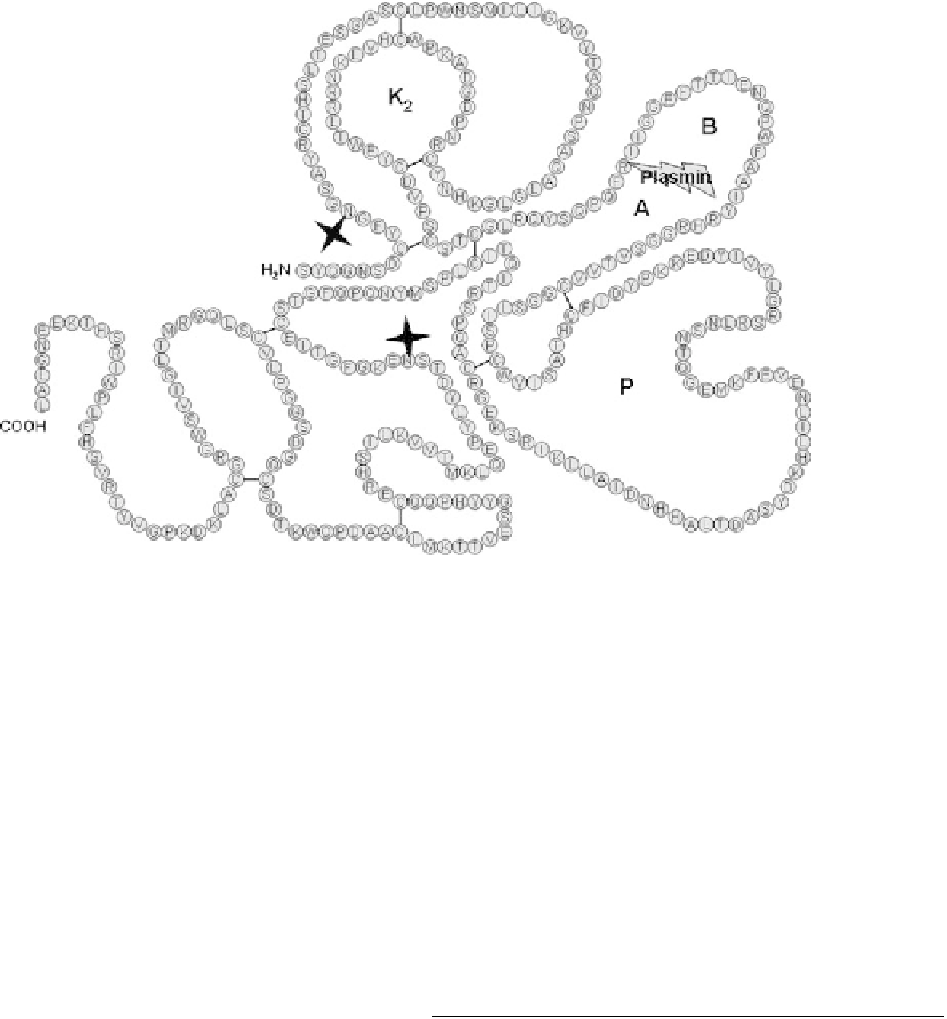
FIGURE 33.2
Primary sequence of amediplase. The plasmin cleavage site is indicated and splits
amediplase into an A chain and a B chain. The diamonds denote positions of N-linked oligo-
saccharide; K2, kringle-2 domain; P, serine protease domain.
which culture supernatant is removed periodically, retaining
the cells in the fermentor. Amediplase is recovered and
purified from the supernatant “harvest.” Each cycle yields
up to 12 of such “harvests.”
Currently, the biotechnological process is characterized
by two fermentation steps following initial expansion of
the seed in stationary flasks and spinners. The purification
consists of four chromatographic steps, low pH viral
inactivation, ultrafiltration/diafiltration followed by a final
filtration step including a viral removal. Transfected
CHO cells grow in a nutrient medium containing no
antibiotics.
Amediplase drug substance is stable at
fivefold higher than amediplase by weight. The others
excipients, succinic acid and sodium hydroxide, are present
to buffer and to adjust the formulation at a pH compatible
with amediplase stability.
One vial amediplase 100mg (powder for injection)
contains
Amediplase
100.00mg
Sucrose
500.00mg
Succinic acid
8.86mg
qs to pH 4.0
a
Sodium hydroxide
qs
b
Nitrogen
20
C for at least
24 months and at 4
C for at least 4 weeks. The quality of the
amediplase drug substance in terms of assay, potency, and
purity (in particular the absence of endotoxins, contami-
nants, and adventitious agents as bacteria, mycoplasma, and
viruses) complies with the EEC requirements for bio-
technological products.
a
theoretical value 1.35mg/vial.
b
used for pre-venting.
For reconstitution of the lyophilized product, 10mL
water for injections (WfI) is prescribed.
The stability has been tested using a bracketing approach.
A 50-mg as well as a 120-mg amediplase containing formu-
lation was investigated. The stability data support a shelf life
of 24 months for both formulations indicating a comparable
shelf life for the 100-mg strength. However, a new stability
study according to ICH has been started for the 100-mg
formulation. The final product should be stored not above
25
C. After reconstitution,
33.2.3 Drug Product
Amediplase is available as lyophilized enzyme in vials
containing 100mg of active principle. Additional excipients
are present in the lyophilized product: sucrose, succinic acid,
and sodium hydroxide. Sucrose serves as a bulking agent
and cake stabilizer and it is present at a concentration
the drug product has to be
administered immediately.

Search WWH ::

Custom Search