Biomedical Engineering Reference
In-Depth Information
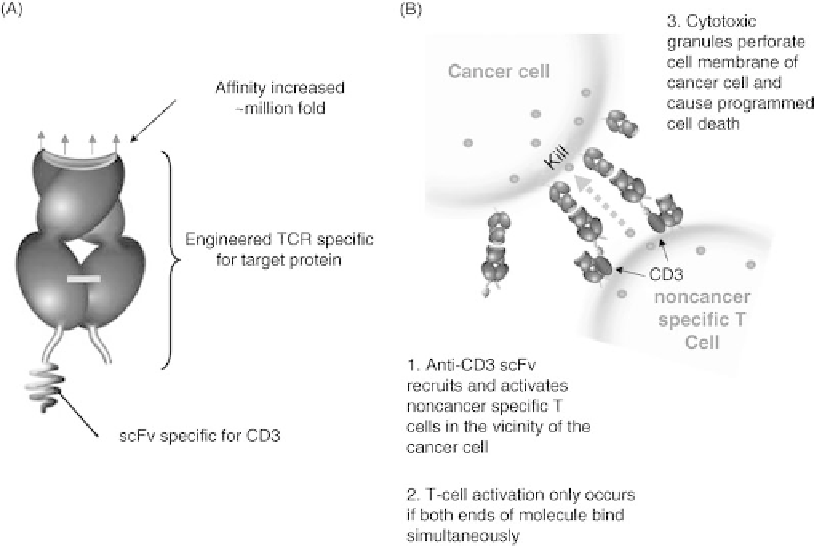
FIGURE 32.4
Redirection of T cells against cancer with ImmTAC. (A) An ImmTAC is a fusion
protein comprising a picomolar affinity mTCR, specific for a chosen cancer-related peptide-MHC,
and an anti-CD3 scFv. (B) The mTCR component binds very strongly to the ligand on a target cell,
while the anti-CD3 moiety activates a proximal T cell independently of its original specificity. The
effect of this redirection is the killing of the engaged cancer cell.
such fusion, ImmTAC-NY-ESO, for which the antigen is
strongly associated with several cancers (Table 32.1). The
use of biotinylated mTCRs is an effective tool for con-
firming epitope presentation as low as 10-50 epitopes per
cell, on both cell lines and fresh tumor biopsies [26]. While
the algorithms designed to predict peptide-MHC presenta-
tion provide valuable clues, high-affinity mTCRs can
confirm the actual processing and presentation of these
epitopes in vivo. In Figure 32.5A, NY-ESO-1 presentation
was quantified on the IM9 cancer cell line and found to be
in the range of 2-13 epitopes per cell. Despite this low
level of antigen presentation, ImmTAC-NY-ESO was
potent and highly specific in activating CD8
target cell and the T cell (top line in figure 32.5B, left); this
feature is crucial for avoiding systemic toxicity when
applied as a therapeutic. Results from cell killing assays
(Figure 32.5B, right) illustrate that the ImmTAC-activated
CTLs were not only activated but also capable of effi-
ciently destroying the target cells.
To investigate the importance of mTCR antigen-binding
affinityforpotency,anumberofImmTACsbasedonNY-
ESO mTCRs, whose affinities varied significantly, were
produced (Figure 32.5C, top). All these variants activated,
in a dose dependent-manner, unstimulated purified CD8
þ
T cells in the presence of IM9 targets (Figure 32.5C,
bottom). As anticipated, however, the ImmTACs based
on the mTCRs of higher affinities were progressively
more potent, achieving a profound effect at concentrations
as low as 10pM in the case of the highest affinity. This
highlights the importance of affinity maturation, and dem-
onstrates the capability of mTCRs as targeting molecules.
Down-regulation of MHC expression is a well-documented
mechanism by which cancer cells evade immune surveil-
lance [24,32]. Thus, to be able to eradicate tumors, an
immunotherapy needs to be effective in the presence of low
levels of peptide-MHC. The A375 cancer cell line, which
expresses MAGE A3, exhibits very low levels of HLA-A1
(also positive by genotype, Figure 32.6 top) MHC I (Figure
CTLs mixed
with IM9 cells (Figure 32.5B, left). The interferon-
g
(IFN-
g
) release assay (Figure 32.5B, left) illustrates that, while
the ImmTAC was showing efficacy at concentrations as
low as 10pM, the stimulation was specific because it was
dramatically reduced when the corresponding nonfused
mTCR was added as a competitor (middle line in Figure
32.5B, left in Figure 32.5B). No CTL activation was
observed with an irrelevant cell line lacking the expression
of NY-ESO antigen but containing the correct HLA-type,
A2 (bottom line in figure 32.5B, left in Figure 32.5B).
Importantly, this illustrates that the ImmTAC only acti-
vates when a multivalent contact is established between the
þ
Search WWH ::

Custom Search