Biomedical Engineering Reference
In-Depth Information
IA preserved the antiviral activity even when incorpo-
rated into HDLs. Purified HDLs containing IA promoted
STAT-1, -2, and -3 phosphorylation in a manner comparable
to the same antiviral units of recombinant IFN-a. In vivo, the
enhanced stability of IA in circulation allows a greater
antiviral activity reflected in an increase in the percentage
of mice that survive a lethal challenge with encephalomyo-
carditis virus.
a subcutaneous injection of CT-26 cells. The adjuvant
treatment with pIA, but not with pIFN, was associated
with significant protection against tumor growth as com-
pared to control group given pApo.
IFN-a has been shown to induce activation-dependent cell
death in lymphocytes [67,68]. Since data suggested that IA
might be more effective than IFN-a in promoting the expan-
sion of stimulated T cells, the lymphocyte proliferation and
viability following T-cell stimulation with anti-CD3 and anti-
CD28 in the presence of IFN-a or the same antiviral units of
HDL-IAwas analyzed. Flow cytometry analysis of stimulated
lymphocytes showed that the number of large blast cells was
markedly reduced in the presence of IFN-a, while valueswere
similar to controls when HDL-IAwas added to the culture. In
these experiments, the lymphocyte proliferation was assessed
by CFSE dilution and lymphocyte death by 7-AAD incorpo-
ration. Both cell proliferation and cell death were similar in
control wells and in wells containing HDL-IA. In contrast, in
the presence of IFN-a, lymphocyte proliferation was reduced
and the number of nonviable lymphocytes was greatly
increased. These data are consistent with the notion that
preservation of viability of activated T cells may underlie
the higher effectiveness of IA in boosting T-cell immunity.
As mentioned, IA is transported in plasma bound to
HDLs, which act as nanocarriers for the fusion protein,
enabling the interaction of IA with both SR-BI and the
IFN-a receptor. After binding to SR-BI, HDLs mediate
the uptake of cholesteryl esters and phospholipids from
the cells and promote cytoprotective functions by mecha-
nisms imperfectly understood involving multiple interac-
tions between HDLs (lipid or protein moiety) and cell
surface receptors [69]. SR-BI is expressed at low levels in
a wide variety of cells, and at high levels in liver, adrenal
glands, ovaries, testis, intestinal cells, phagocytes, and
endothelial cells [38,70]. To investigate whether combined
interaction with SR-BI and IFN-a receptor was important in
mediating the superior immunostimulatory activity of IA as
compared to IFN-a,anin vivo killing against b-galactosi-
dase epitope was performed, following a vaccination with
29.1.6 Unexpected Properties of the Fusion Protein
of Interferon-
a
and Apolipoprotein A-I
A clear difference between IA and IFN-a was observed
when cell viability and cytotoxicity were analyzed. It is
noteworthy that IFN-a at the dose used for signaling experi-
ments caused an increase in cell death, while the same
antiviral units of HDL-IA caused no cytotoxic effect, cell
viability being similar to that of control wells.
In keeping with the differential effects of IA and IFN-a
on cell proliferation, those two molecules were found not to
be comparable in their actions on the hematopoietic system.
Thus, 3 days after plasmid injection, platelets and leukocytes
were significantly higher in IA-treated mice than in IFN-a or
albumin-IFN-a treated mice (Figure 29.4).
IA also differs from IFN-a and from albumin-IFN-a
because of its immunomodulatory properties. In vivo killing
assays against a Balb/c immunodominant b-galactosidase
epitope were performed in mice, which 7 days previously
had been given a hydrodynamic coinjection of a plasmid
encoding LacZ together with pIFN, pIA, pALF, or pApo.
These studies showed that the group treated with pIA
exhibited a cytolytic activity significantly greater than the
other three groups. Differences were also observed between
pIFN and pIA in the induction of protective immunity in a
murine model of vaccination against CT-26 colon cancer.
Vaccination was performed by subcutaneous administration
of peptide AH-1 (which corresponds to the tumor-immuno-
dominant epitope) in combination with hydrodynamic injec-
tion of pApo, pIFN, or pIA. Ten days later, animals received
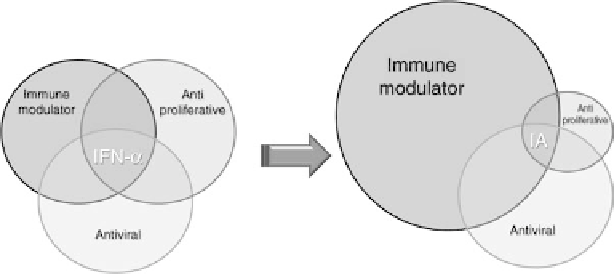
FIGURE 29.4
Scheme of the differential effects between interferon-a and the fusion molecule of
interferon-a and apolipoprotein A-I.
Search WWH ::

Custom Search