Biomedical Engineering Reference
In-Depth Information
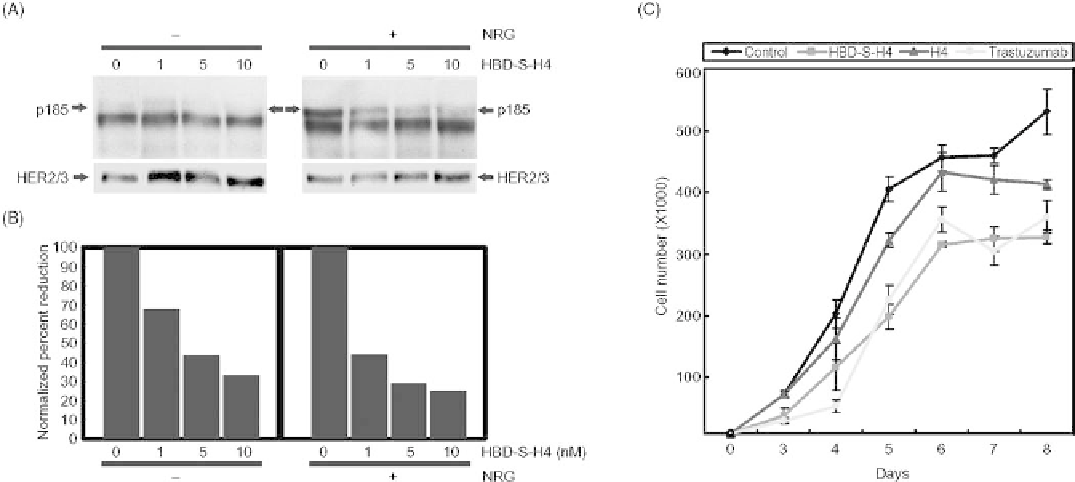
FIGURE 27.6
HBD-S-H4 blocks the growth of malignant human breast cancer MCF10CA1 cells
through disruption of autocrine and paracrine NRG signaling. (A) Increasing concentrations of HBD-
S-H4 were added to MCF-10CA1 breast cancer cells for 45min without or with 75 pM recombinant
NRG. The top panel shows that HBD-S-H4 blocked both autocrine (without added NRG) and
paracrine (with NRG) HER2/3 receptor phosphorylation (p185). The lower panel shows the same
Western blot reprobed with HER2 and HER3 antibodies. (B) Quantitation of the p185 signal
normalized to HER2/3 levels is shown. (C) Growth curves of MCF10CA1 cells were compared
without or with HBD-S-H4, H4, or trastuzumab (Herceptin) treatment. HBD-S-H4 (1 nM) markedly
reduced the proliferation rate of these cancer cells compared to an equal molar amount of H4, and was
comparable to treatment with trastuzumab. Source: This research was originally published in J. Biol.
Chem. Reference 79.
is unique in that it is an easily detachable, stand-alone C2
immunoglobulin domain that can retain its targeting speci-
ficity both in vitro and in vivo when fused to another
polypeptide. Early data suggest that this completely human-
ized fusion protein may be promising as an anti-NRG1
biopharmaceutical in diseases ranging from chronic pain
to cancer. It serves a good example on how it is possible to
improve therapeutics by targeting biopharmaceuticals to
where they are needed with minimal toxicity to other tissues
and thus resolves one of the most important obstacles in the
development of biological therapeutics. It is tempting to
further speculate that subtle modifications in this HBD or
other potential HBD sequences could modify its interactions
with HS sufficiently to change tissue-specific targeting
specificity that could lead to targeting vectors for tissues
other than where NRG1 goes.
2. Yao B, Rakhade SN, Li Q, Ahmed S, Krauss R, Draghici S, et
al. (2004) Accuracy of cDNA microarray methods to detect
small gene expression changes induced by neuregulin on breast
epithelial cells. BMC Bioinformatics 599.
3. Thoenen H, Sendtner M. (2002) Neurotrophins: from
enthusiastic expectations through sobering experiences to
rational therapeutic approaches. Nat. Neurosci. 5(Suppl),
1046-1050.
4. Aszodi A, Legate KR, Nakchbandi I, Fassler R. (2006) What
mouse mutants teach us about extracellular matrix function.
Annu. Rev. Cell Dev. Biol. 22, 591-621.
5. Bulow HE, Hobert O. (2006) The molecular diversity of
glycosaminoglycans shapes animal development. Annu. Rev.
Cell Dev. Biol. 22, 375-407.
6. Toole BP. (2004) Hyaluronan: from extracellular glue to
pericellular cue. Nat. Rev. Cancer 4(7), 528-539.
7. Turnbull J, Powell A, Guimond S. (2001) Heparan sulfate:
decoding a dynamic multifunctional cell regulator. Trends Cell
Biol. 11(2), 75-82.
8. Iozzo RV. (1998) Matrix proteoglycans: frommolecular design
to cellular function. Annu. Rev. Biochem. 67, 609-652.
9. Kramer KL, Yost HJ. (2003) Heparan sulfate core proteins in
cell-cell signaling. Annu. Rev. Genet. 37, 461-484.
REFERENCES
1. Li Q, Ahmed S, Loeb JA. (2004) Development of an autocrine
neuregulin signaling loop with malignant transformation of
human breast epithelial cells. Cancer Res. 64(19), 7078-7085.
Search WWH ::

Custom Search