Biomedical Engineering Reference
In-Depth Information
As a first step, we generated a series of fusion proteins to
ask what the optimal arrangement is between the HBD fused
to H4 for both heparin binding and NRG1 antagonism
(Figure 27.3). The constructs we tested placed the HBD
either N-terminal or C-terminal to H4, and then compared
these to H4 expressed alone. Figure 27.3a shows a heparin
column-binding assay that uses increasing concentrations of
NaCl to elute bound fusion proteins as a means to screen for
heparin-binding affinity. While both N- and C-terminal
fusion proteins could bind to the column, the N-terminal
HBD fusion bound heparin only when it was separated from
the H4 domain by a spacer region. The spacer domain used
was the endogenous, glycosylated spacer domain from
NRG1 that normally separates the HBD from NRG1's
EGF-like domain. In fact, this fusion protein (HBD-S-H4)
was not only optimal for heparin binding but also the most
potent fusion protein for blocking NRG1 activity (Figure
27.3b). The spacer domain may be required to allow an
optimal protein conformation necessary for heparin binding
or to prevent steric inhibition between the other two
domains. In comparison, the H4 construct without the
HBD, showed little effect blocking receptor phosphoryl-
ation. Once concentrated in the ECM, heparin binding
enables the antagonist to have long-lasting effects that
cannot be removed even after vigorous washing steps to
remove any proteins not bound to cells (Figure 27.3c). All
these findings further demonstrate that, even when removed
from its native protein, NRG1's isolated HBD retains high
heparin-binding affinity when fused to another protein and
can be effectively used to generate heparin-binding fusion
proteins with enhanced and sustained activity.
Further experiments provided additional evidence of the
importance of HS binding for the HBD-S-H4 fusion protein.
Using wild-type cells and mutant cells that lack the ability to
synthesize HS, we found that HBD-S-H4 adheres only to
those cells that produce HS in the ECM, whereas the H4
control protein (without the HBD) adheres to neither (Figure
27.4a, b). Furthermore, heparinase treatment of wild-type
cells (that selectively degrades cell-surface HS) significantly
decreases HBD-S-H4 binding. Finally, a heparin-coated
plate was used to determine that the Kd of HBD-S-H4
was 60 nM (Figure 27.4c). All these results demonstrate
that the increased potency of HBD-S-H4 fusion protein is in
part due to its ability to interact with specific HS in the
matrix and concentrate on cell surfaces of cells that express
HSPGs.
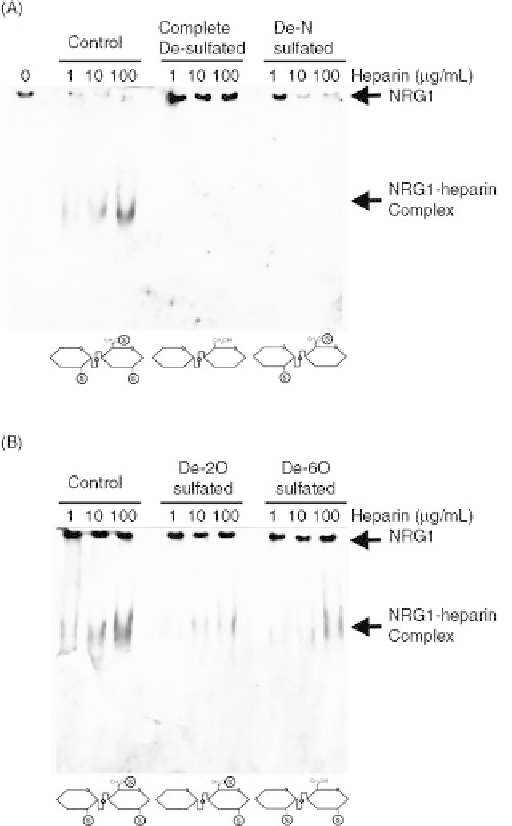
FIGURE 27.2
N-Sulfation is more important for heparin-NRG1
binding than 2-O- and 6-O- sulfation. Parallel gel shift assays were
performed using the following: (A) Fully sulfated heparin, com-
pletely desulfated heparin, and De-N-sulfated heparin or (B) Fully
sulfated heparin, De-2-O-sulfatd heparin, and De-6-O-sulfated
heparin at 0, 1, 10, and 100
m
g/mL. Completely desulfated and
De-N-sulfated heparin were not able to bind and shift NRG1,
whereas De-2-O- and De-6-O- sulfated heparins shifted NRG1
better than De-N-sulfated heparin but less than fully sulfated
heparin. Schematic representations of each of the modified hepa-
rins are shown at the bottom of each gel with sulfate group marked
with an S. Source: This research was originally published in J. Biol.
Chem. Reference 68.
epidermal growth factor receptor 4 (HER4/H4). The HER4
ectodomain has high affinity for NRG1's EGF-like domain
and can thus be used as a dominant negative NRG1 antago-
nist. By fusing HER4 to NRG1's HBD, the antagonist
should be targeted to the same HS rich cell surfaces that
bind NRG1 and therefore effectively disrupt NRG1 signal-
ing [79].
27.10 TISSUE TARGETING AND THERAPEUTIC
EFFICACY OF A HEPARIN-TARGETED NRG1
ANTAGONIST FUSION PROTEIN
Given the retained high-affinity heparin binding of the HBD-
S-H4 fusion, we next asked whether the in vivo targeting
Search WWH ::

Custom Search