Biomedical Engineering Reference
In-Depth Information
20.0
2005
2006
2007
2008
2009
2010
18.0
16.0
14.0
12.0
10.0
8.0
6.0
4.0
2.0
0.0
Strong growth
Slow growth
FIGURE 1.4
Annual sales of protein drugs in the United States. The two classes of proteins with a
high annual growth rate are monoclonal antibodies (mAB) and hormones. Over the last 4 years, the
growth of sales for fusion proteins was minimal, whereas the sales for growth factors even declined.
of nature's building blocks. Many concepts have been
heavily influenced by the “magic bullet paradigm” that
has been described more than a century ago, but took until
now to realize it with artificial recombinations. These
novel fusion proteins combine hitherto unrelated function-
alities into a single molecule. Several have passed approval
from regulatory authorities and many of those currently
beinginclinicaltrialswillreachthemarketsoon.Sofar
fusion proteins have proven as valuable additions to the
arsenal of therapeutic molecules. Still there are many
opportunities where innovative fusion proteins can make
a significant improvement. For instance, many first gener-
ation biopharmaceuticals can benefit from prolonged cir-
culation times. Cancer patients will receive better targeted
and more specific drugs with less systemic toxicity based
on fusion proteins. So far relapsing tumors can be treated
with novel protein drugs that hit two targets simulta-
neously, so overcoming resistance mechanisms. Novel
fusion proteins will make therapies more affordable by
lowering manufacture cost and improve quality of life for
many patients who benefit from longer administration
intervals. However, to fulfill the promises of fusion protein
technology still a number of challenges have to be
resolved.
A major obstacle is the immunogenicity potential that is
always present even in fully human recombination, because at
joint between two molecules will always create a new epitope
which can provoke immune reactions. Reduction of immuno-
genicity and understanding the underlying factors is, therefore,
a key element to guarantee future success of fusion proteins.
Another point to consider is the potential incompatibility
between fusion partners that limit manufacturability. Both
challenges will require intense efforts of protein engineering.
This topic aims to cover the state of the art of fusion
proteins. It presents an overview on the multitude of
possibilities to design novel protein drugs while balancing
between proven concepts and new ideas that have not
reached the clinic yet. The topic is structured into three
larger parts. First general issues and concepts are dis-
cussed before in the second part examples on the three
categories (t
1/2,
toxicity, and targeting) are presented.
Finally novel concepts and the rising class of multispecific
antibodies are described. I hope this topic will inspire the
reader and create enthusiasm for the exciting topic of
fusion proteins.
REFERENCES
1. Cohn EJ, Oncley JL, Strong LE, Hughes WL, Armstrong SH.
(1944) Chemical, clinical, and immunological studies on the
products of human plasma fractionation. I. The characterization

















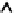



















































Search WWH ::

Custom Search