Biomedical Engineering Reference
In-Depth Information
CPP
(C)
(A)
HO
Purification tag
Protein
O
Disulfide bond
H
2
N
′
′
N
C
S
(
D
)
O
S
Peptide bond
′
R
R
N
H
Maleimide
group
O
NH
2
O
Sulfur-carbon bond
NH
OH
CPP
(B)
Purification tag
Protein
O
S
′
N
′
C
H
2
N
Cys
teine
O
O
Peptide bond
HO
′
R
R
N
H
FIGURE 26.1
Methods for producing cell-penetrating fusion proteins. (A and B) Schematic
drawing of the segment of DNA that is inserted to a vector for expression of the most common
construct for CPP-fusion proteins, in the resulting fusion protein the CPP is either flanked by two
glycine residues to allow for free rotation or there are no additional amino acids between the CPP and
the protein. (C and D) Illustration of the two available methods for using cysteines to create covalent
linkages between CPPs and proteins.
expression in E. coli. A vector containing the gene for a
protein and either N- or C-terminal DNA encoding for a CPP
is generated by inserting cDNA for the protein into an
existing vector such as the pTAT-HAvector [59]. This vector
contains an N-terminal hemagglutinin (HA)-tag and a
hexahistidine (His-6)-tag followed by the TAT peptide.
The two tags allow for standardized purification utilizing a
tandem strategy for affinity-based chromatography. This
vector was first described by Nagahara [52]. The vector has
since then been extensively used for creating fusions of
TAT and various proteins
thrombin cleavage site (SGLVPRGS), TAT protein trans-
duction domain (PTD), HA-tag, and Bcl-xl, and the resulting
vector was designated as pET-PTD-HA-Bcl-xl. Expression
was carried out in E. coli and the purification was performed
using a Ni-NTA superflow agarose column. The His6-tag
was removed by thrombin digestion and a second round
through the Ni-NTA column removed the His6-tag.
On a side note, there have been indications that it may
make a difference on which terminus (N or C) of the protein
the CPP is fused. In a study where VP22 was fused both N-
and C-terminally with p53, the N-terminally fused construct
showed no activity, whereas the C-terminal showed a sixfold
increase [55]. This is something that perhaps should be
considered and evaluated for each protein and peptide
combination.
in for
example
[4,20-
22,24,31,33,41,44,45,56,59-77].
Another example, in this case, novel vector, created for a
specific application, is a vector expressing a VP22-eGFP
fusion protein [32]. This vector was produced using a
commercially available kit (Invitrogen's Voyager Protein
Production Kit),
in accordance with the manufacturers
26.3.3 Disulfide Bond
instructions.
A third example is the fusion between B-cell lymphoma-
extra large (Bcl-xl) and TAT [19] where the authors started
by isolating the Bcl-xl by PCR from a rat brain cDNA
library, subcloned into the pSPORT1 vector (Invitrogen, San
Diego, CA), amplified, purified, and verified it (with
sequencing analysis). They then reconstructed the ET-30
expression plasmid to allow the generation of hemagglutinin
(HA) tagged Bcl-xl-TAT fusion protein that contained the
TAT-peptide sequence. The final cDNA (which replaced the
enterokinase cloning site in the ET-30 plasmid) encodes
peptide sequences
A method that employs the disulfide bond between cysteines
or a carbon-sulfur bond between a carbon and a sulfur, that
is, between a cysteine and a maleimide group, can also be
used to produce CPP-fusion proteins. For example, a Fab
antibody fragment labeled with Iodine 125 and three differ-
ent CPPs (REV, TAT, and ANP) have been conjugated by
synthesizing the peptides with an additional cysteine and
then allowing these peptides to react with the radio-labeled
antibody fragment treated with N-(6-maleimidocaproyloxy)
succinimide ester (EMCS) [78]. The functional groups are
illustrated in Figure 26.1.
in the following order: His6-tag,









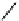
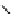






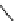





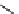




















Search WWH ::

Custom Search