Biomedical Engineering Reference
In-Depth Information
binding to other receptors and was fused to the C-terminal
end of
b
-glucuronidase (GUS). This fusion protein was
effective in clearing pathological lysosomal storage in a
mouse model of mucopolysaccharidosis type VII. Interest-
ingly, the GILT variant of GUS could also reach osteoblasts
that could not be treated with the original enzyme [52].
Fusion protein based strategies for LSD are summarized in
Figure 25.3.
A receptor-independent enzyme delivery to treat LSD
utilizes the protein transduction domain of the human
immunodeficiency virus (HIV) transacting activator of tran-
scription (TAT) protein. GUS connected to the 11 amino
acids of TAT at the C-terminus is internalized by adsorptive
endocytosis in a receptor and glycosylation independent
manner. The underlying mechanism is dependent on a
positive net charge of the molecule that can adhere to
negatively proteoglycans at the cell surface. Overall, the
GUS-TAT fusion was less efficiently uptaken by this trans-
port than via the Man6-P receptor, but was more efficiently
delivered to the kidney [53]. The independence of Man6-P
receptors could also be demonstrated with glucocerebrosi-
dase-TAT fusions that were delivered to fibroblasts lacking
the Man6-P receptors [54]. TAT as fusion partner was also
successfully employed to restore lipoamide dehydrogenase
(LAD; also known as E3) deficiency. TAT connected to the
amino terminus of LAD was delivered to the mitochondria
of E3-deficient mice in liver, heart, and brain [55].
25.3.2 Cell Penetrating Peptides
The aforementioned TAT peptide belongs to the family of
cell-penetrating peptides (CPP). A number of these peptides
have been used as targeting modules of fusion proteins. One
example is
the EPO-TAT fusion for brain delivery.
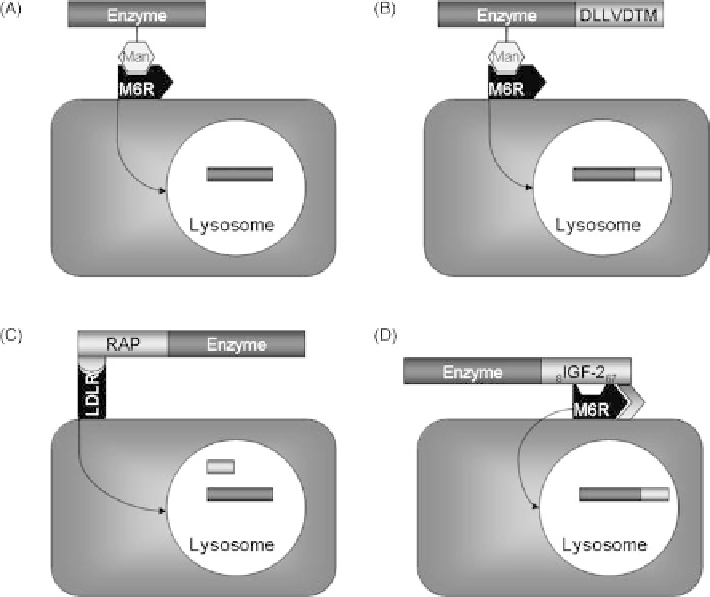
FIGURE 25.3
Enzyme replacement strategies for lysosomal targeting. Many lysosomal storage
diseases can be treated by enzyme replacement strategies. (A) Originally lysosomal enzymes contain
a specific glycosylation signature (Man) that mediates their transfer to the lysosome via mannose
receptors (M6R). (B) Enzymes can be artificially mannosylated by attaching a short peptide sequence
to their C-terminus. When expressed in plants, proteins with this tag are translocated to the storage
vacuole where they are mannosylated. (C) Instead of the mannose receptor, the low-density
lipoprotein (LDLR) receptor can be utilized as well. Ligands of LDLR are transferred to the
lysosome. The receptor-associated protein (RAP) binds with high affinity to LDLR but is cleaved off
from its payload in the lysosome. (D) The cation-independent mannose-6-phosphate receptor does
also recognize of insulin-like growth factor 2 (IGF-2). A truncated form of IGF-2 fused to the
C-terminal of an enzyme targets the fusion protein to the lysosome. This is called glycosylation-
independent lysosomal targeting.
Search WWH ::

Custom Search