Biomedical Engineering Reference
In-Depth Information
disruption of micro-RNAs, resulting in the suppression of
protein synthesis [42].
drugs are universally cytotoxic but achieve specificity by
targeting receptors that are only displayed on the surface of
tumor cells (immunotoxins) [1]. This can be achieved using
specific targeting moieties such as monoclonal antibodies, as
in the case of gemtuzumab ozogamicin (Mylotarg
1
) for the
treatment acute myeloid leukemia [48]. Unfortunately, these
approaches are less effective in practice due to the emer-
gence of clinical resistance, as well as significant off-target
effects resulting from, inter alia, the expression of the tumor-
associated antigen on nonmalignant cells, albeit at lower
levels [49]. Mylotarg was withdrawn voluntarily from the
market in 2010 for this reason.
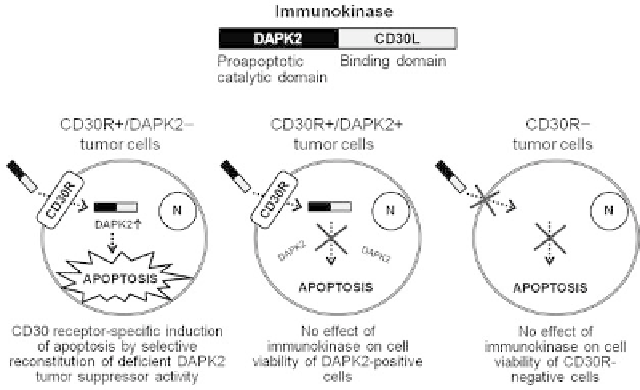
The immunokinase fusion protein strategy combines the
advantages of the targeted delivery and targeted activity
approaches discussed above, therefore reducing the potential
for off-target effects to very low levels. The concept has been
discussed for DAPK1, which is downregulated in chronic
lymphocytic leukemia (CLL) cells, leading to speculation
that the restoration of DAPK1 activity in CLL cells might
increase their sensitivity to apoptosis [9,35]. In our proof-of-
principle study with DAPK2, the same goal is achieved by
targeting Hodgkin lymphoma cells with a functional tumor
suppressor protein that is only downregulated in Hodgkin
lymphoma cells (i.e., its delivery to other cells would have
no impact) [7] (Figure 21.3). As discussed above, we
determined that DAPK2 expression is epigenetically
silenced in Hodgkin lymphoma L540 cell lines, so we set
out to determine whether the selective reconstitution of
DAPK2 catalytic activity in these cells could induce apo-
ptosis. We therefore created immunokinase fusion proteins
comprising the catalytic domain of human DAPK2 joined by
a linker to the extracellular domain of human CD30L [3] or
the murine anti-CD30 single chain variable fragment anti-
body Ki-4 [50]. CD30 is an attractive candidate target for
immunotherapy because
21.3.2 Nontargeted Reactivation of Specific Protein
Kinases
Because demethylating drugs lack specificity and can
reactivate dormant oncogenes, strategies have been devel-
oped to target individual tumor suppressor genes, including
those encoding protein kinases [43]. In the case of DAPK1,
the expression of DAPK1 cDNA in stably transfected Lewis
carcinoma cells prevented metastasis in a murine model of
lung carcinoma by promoting apoptosis [12] and the over-
expression of DAPK1 and DAPK2, but not of their catalyti-
cally inactive mutants K42A and K52A, induced apoptosis
in several transfected malignant cell lines through a range of
apoptotic and/or autophagic signals [23,26,27]. Concep-
tually, these strategies are similar to those applied to
reactivate the retinoblastoma protein [44] and p53
[45,46]. In all cases, however, the expression construct
was delivered using a plasmid or viral vector, which is
unsuitable for specific tumor targeting in vivo.
21.3.3 Targeted Reactivation of Specific Protein
Kinases Using DAPK2-Based Immunokinases
The disadvantages of systemic delivery can be overcome
through the development of therapeutic modalities that are
targeted specifically to tumor cells, thus avoiding off-target
effects on normal cells. Certain drugs aspire to this objective
by targeting proteins that are solely expressed in cancer
cells, meaning that all cells receive the drug but only cancer
cells should be affected. An example is imatinib, which
blocks an aberrantly expressed serine/threonine kinase
activity in chronic myelogenous leukemia cells [47]. Other
it
is
internalized efficiently
FIGURE 21.3
Schematic diagrams showing principle of operation of immunokinase fusion
proteins for dual-targeted cancer treatment.
Search WWH ::

Custom Search