Biomedical Engineering Reference
In-Depth Information
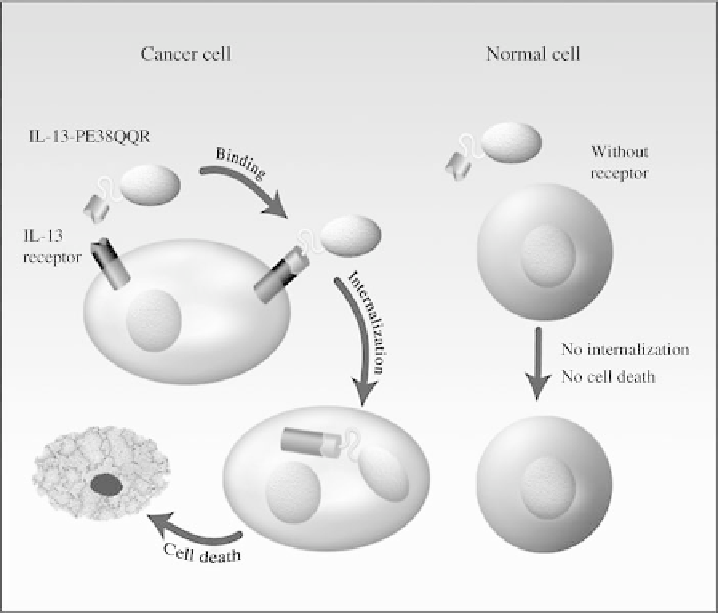
FIGURE 20.2
Schematic representation of the mode of action of the recombinant fusion toxin IL-
13-PE38 (IL-13-PE38QQR). IL-13 receptor (IL-13R-
a
2 subunit) is a tumor-specific protein in
human malignant glioma cells. Immune cells, endothelial cells and normal glia and neurons express
none or very low amounts of IL-13R. IL-13-PE38 is highly selective and potent in killing human
GBM cells by binding to IL-13R, but does not kill normal cells, which are IL-13R-negative.
which would require
8 months to diffuse 1 cm. Blood
perfusion was measured in the infused hemisphere by
99
Tc-
HMPAO-SPECT and showed no significant
concentrations of drug. All clinical trials with targeted toxins
have adopted CED as the delivery mode of choice.
reduction
(
5%) compared to the control hemisphere. Histology
demonstrated only mild gliosis immediately surrounding
the needle tract. This study showed that CED could distrib-
ute a high molecular weight protein over centimeter dis-
tances in nonhuman primate brain and could do so
reproducibly and safely [31].
In nude mice with intracerebral glioma receiving an
intratumoral bolus injection of IL-13-PE38, drug distribu-
tion was maximal at 1 h after injection and the volume of
distribution was 19.3
<
20.4 PRECLINICAL AND CLINICAL STUDIES
WITH TARGETED TOXINS
20.4.1 IL4-PE (NBI-3001)
The chimeric recombinant fusion protein IL-4-PE (proprie-
tary designation of IL-4-PE, originally owned by Neurocrine
Inc., S. Diego, CA) is composed of circularly permuted
interleukin-4 (IL-4) and a truncated form of P. aeruginosa
exotoxin (PE) A [18,19,23]. PE is a 66-kDa protein with
three domains: Ia/Ib, II, and III. The N-terminal domain Ia
binds to the
a
2
-macroglobulin receptor (
a
2
-macroglobulin
receptor/low-density lipoprotein receptor-related protein,
LRP), and the ligand-receptor complex undergoes recep-
tor-mediated internalization and processing of the toxin.
When the domain Ia is removed, the resulting molecule
(termed PE-40) retains its translocation function and elon-
gation factor 2 (EF-2) inhibition properties, but is unable to
5.8 mm [32]. Intratumoral infusion
of IL-13-PE38 via CED in the same glioma model resulted
in better distribution than intratumoral bolus administration,
with the drug remaining in the brain for 6 h after the
treatment [33].
Because human GBM is a neoplastic disease not metas-
tasizing outside the CNS, local delivery of therapeutic
agents such as toxins via CED seems to be the best approach
to circumvent the limitations of the blood-brain barrier
(BBB) and to increase therapeutic efficacy by high local
Search WWH ::

Custom Search