Biomedical Engineering Reference
In-Depth Information
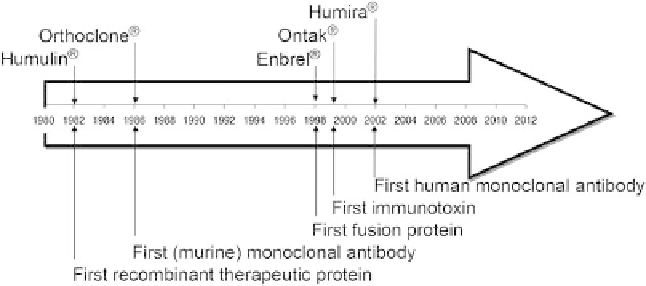
FIGURE 1.1
Milestones of recombinant therapeutic proteins.
the name Humira
1
in 2002 [5]. The milestones for recom-
binant therapeutic proteins can be seen in Figure 1.1.
Interestingly, preventing the activity of TNF-
a
was also
the goal for the first fusion protein, Etanercept. It consists of
the TNF-
a
receptor attached to a sequence encoding the Fc
portion and hinge region of an IgG1 heavy chain. This drug
has been marketed under the name Enbrel
1
since 1998 and
is the best selling fusion protein till date [6]. The two
extraordinarily successful drugs Humira and Enbrel can
serve as prototype for their molecule classes, exemplifying
the different ways to address the same target and present a
typical case of competition between antibodies and fusion
proteins in the market.
Recently, a review classified therapeutic proteins accord-
ing to their pharmacologic activity to (a) replace a deficient
or abnormal protein, (b) augment an existing pathway,
(c) provide a novel function or activity, (d) interfere with
a molecule or organism, or (e) deliver a payload such as a
radionuclide, cytotoxic drug, or protein effector [12].
However, this classification is not fully suitable for the
scope of this topic. Most fusion proteins serve three major
purposes that can be summarized under the triple T (T3)
paradigm: (a) t
1/2
(half-life), (b) targeting (or binding), or
(c) toxicity (cell killing). Of these three elements, at least two
are simultaneously present in fusion proteins (Figure 1.2).
Antibodies as natural molecules combine all three aspects in a
singlemolecule. However, antibody derivatives, fragments, or
domains have also been used extensively as building blocks
for fusion proteins, hence constituting a large part of the
portfolio of proteins discussed here, and thus deserving their
own category. The main functionality of antibodies, the
binding with high affinity and selectivity to a specific epitope,
has been reproduced in a number of nonantibody scaffolds
that can either be used as single module or by combining two
units with different specificity [13]. These molecules together
with other bi- or multifunctional therapeutics that do not fit to
the T3 categories are classified into the group of novel
artificial molecules that is discussed in Part IIIa of this topic.
A very practical classification is proposed by the authors
of Chapter 3 about “Structural Aspects of Fusion Proteins
Determining the Level of Commercial Success” in this topic.
They suggest sorting fusion proteins based on one of three
different functional groups: activity, targeting, or half-life, of
which the latter two can be summarized under delivery
agents, thus being able to define a two-dimensional land-
scape of fusion proteins. This is quite similar to a previous
scheme using the combination of an effector fragment
together with a molecular recognition part as building blocks
for fusion proteins [14].
But why should we deal at all with fusion proteins?
Several advantages make them very attractive: the combi-
nation of two functionalities in a single molecular entity
1.2 DEFINITIONS AND CATEGORIES
This topic focuses on fusion proteins that are generated by
joining two or more genes by genetic engineering that
originally code for separate proteins. The result is a single
polypeptide with functional properties of both parent pro-
teins. These recombinant proteins are combinations of
unrelated domains not occurring in nature. Excluded from
the content of this topic are multiepitope recombinant
vaccines [7], chemical conjugates [8] naturally occurring
fusion proteins resulting from chromosomal rearrangements
that can be observed in many cancer cells [9] or fusion tags
for affinity purification [10].
Bi or multispecific antibodies are special case that do not
always represent a single polypeptide chain but usually
consist of the combination of heavy and light chains. The
Part IIIb of this topic discusses some non-natural versions
that have more than a single specificity.
The most straightforward classification of these novel
proteins can be based on the functions of their incorporated
domains. Typically, one part serves molecular recognition or
binding, whereas the other part adds certain functionalities
such as extending half-life or stability, cytotoxicity, or novel
targeting or delivery routes [11].
Search WWH ::

Custom Search