Biomedical Engineering Reference
In-Depth Information
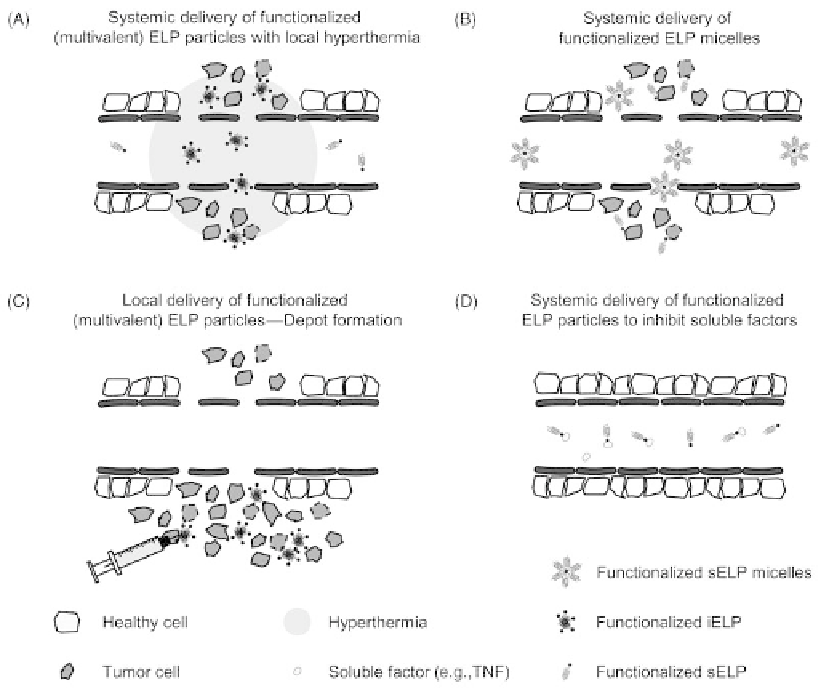
FIGURE 14.3
Delivery strategies and working principles of functionalized ELPs. (A) Targeting of
a functionalized ELP by local hyperthermia to a tumor by the phase transition triggered aggregation
of the ELP in tumor vasculature and passive passing through the leaky tumor vasculature,
functionalized ELP might have multivalent properties. After return to normothermia, the aggregates
might dissolve, generating a large concentration gradient that drives the ELP that dissolves from the
aggregates into the tumor. (B) Functionalization of ELP with a C-terminal hydrophobic drug such as
Paclitaxel, trigger the self-assembly of ELP micelles, which are passing through the leaky tumor
vasculature. (C) Depot formation of functionalized ELP-aggregates (T
t
below body temperature) by
local delivery upon intratumoral injection. (D) Systemic delivery of functionalized ELPs (monomeric
or multivalent) can bind and neutralize soluble factors within the circulation or inflamed tissues
(e.g., anti-TNF, anti-IL-6). Source: Modified from Reference [124].
of autoimmune and chronic inflammatory diseases [65]. The
association of ELP-sTNFRII fusion proteins caused a sig-
nificantly reduced in vitro bioactivity [66]. However, TNF-
mediated effects in cultured astrocytes and microglia or
dorsal root ganglion explants were attenuated by ELP-
sTNFRII-fusions. This is consistent with the anti-TNF
effects of unfused sTNFRII [67]. The same group analyzed
the aggregation kinetics of radioactively labeled ELP.
Aggregated ELPs demonstrated a sevenfold longer perineu-
ral half-life as compared with the soluble ELPs. They
concluded that thermally stimulated aggregation of ELP
fused therapeutics might provide a perineural drug depot
for longevity drug delivery to an inflamed nerve [68].
ELPs were fused to a cell penetrating peptide and a cell-
cycle inhibitory peptide derived from the p21 protein, which
caused a portion of the polypeptide to reach the nucleus [69].
The engineered protein aggregated at temperatures above
39
C and showed enhanced proliferation inhibitory effects
in this stage, thus rendering the protein a thermally respon-
sive carrier that might be targeted to solid tumors by
application of focused hyperthermia [70]. Furthermore,
Massodi and colleagues constructed a fusion protein of
the cell penetrating Tat peptide with ELP, which was shown
to inhibit cell adhesion, spreading, invasion, and migration
of ovarian cancer cells in culture. In vivo, the same protein
exhibited an anti-metastatic potential in an ovarian cancer
Search WWH ::

Custom Search