Biomedical Engineering Reference
In-Depth Information
was cleared rapidly in rabbits, on the other hand, sialic
acid capping of the glycan chains of erythropoietin reduces
the clearance in vivo. Similar observations have been made
with other glycoproteins including orosomucoid and hCG
[5,12,13]. The primary reason for the rapid clearance of the
asialoglycoproteins in previous studies seems to be asialo
(exposed) Galactose (Gal) or GalNAc that lead to uptake by
lectin receptors in the liver [14]. Circulating glycoproteins
usually do not contain O-linked oligosaccharides, and pre-
vious data focused on glycoproteins bearing terminal N-
linked oligosaccharides. Asialoglycoproteins bearing termi-
nal N-linked Gal or GalNAc are internalized by the asialo-
glycoprotein (ASGP) receptor via the clathrin-coated pit
pathway and are delivered to lysosomes for degradation
[15]. The ASGP receptor has been reported to be on the
plasma membrane of parenchymal cells/ hepatocytes.
N-Terminal
—Ser Ser Ser
Ser
Lys Ala Pro Pro Pro
Ser
Leu Prol Ser Pro
Ser
Arg Leu Pro Gly Pro
Ser
Asn Thr Prol lle Leu Pro Gln-
C
—
Te r m i n a l
FIGURE 13.2
Amino acid sequence of hCG carboxyl-terminal
peptide. The positions of O-linked oligosaccharide chains are
indicated. O-linked oligosaccharides are indicated as
.
glycoprotein hormone family (follitropin [FSH], thyrotropin
[TSH], luetropin [LH]) in that it contains a unique 28 amino
acid carboxyl-terminal peptide bearing four O-linked oligo-
saccharide chains (Figure 13.2). Glycoprotein hormones are
composed of two subunits: the
a
-subunit that is common to
FSH, TSH, LH, and hCG and a specific
b
-subunit, which
confers the hormone bioactivity. Subunits assembly is the
rate-limiting step for secretion, receptor binding and bio-
activity of the hormone.
It has been suggested that the O-linked oligosaccharide
chains play an important role in the secretion of intact hCG
from the cell, enhanced bioactivity in vivo and prolonged its
circulating half-life [6]. Deletion of the CTP including the
O-linked oligosaccharide chains from hCG using site-
directed mutagenesis, did not affect assembly of the subunits
or secretion of the dimer from the cell. On the other hand, it
was shown that truncated hCG without the CTP is three
times less potent than intact hCG in vivo [7]. On the other
hand, the O-linked oligosaccharide chains play a minor role
in receptor binding and signal transduction. These findings
indicate that the CTP of hCG
b
and the associated O-linked
oligosaccharides are not important for receptor binding or in
vitro signal transduction but are critical for in vivo bio-
activity and half-life [6]. It was reported that the kidney is
the main site of clearance for glycoprotein hormones. On the
other hand, much less hCG, which contains the CTP associ-
ated with the four O-linked oligosaccharide chains, is
cleared by the kidney [8,9]. Other studies indicated that
sialic acids attached to the end of oligosaccharide chains,
play an important role in the survival of glycoproteins in the
circulation [10]. It has been suggested that more negatively
charged forms of glycoprotein hormones have longer half-
lives, which may be related to a decrease glomerular filtra-
tion [11]. Thus, it was hypothesized that the presence of CTP
with its sialylated O-linked oligosaccharides may prolong
the circulating half-life of the hormone secondary to a
decrease in renal clearance.
Previous studies have shown the important role of
sialylation in the pharmacokinetics and distribution of gly-
coproteins. It was demonstrated that asialoceruloplasmin
13.3 DESIGNING LONG-ACTING AGONISTS
OF GLYCOPROTEIN HORMONES
One major issue regarding the clinical use of glycoprotein
hormones is their relatively short half-life in vivo. To address
this issue, the CTP sequence of hCG
b
containing four
recognition sites of O-linked oligosaccharides was fused to
the carboxyl-terminal of hFSH
b
, hTSH
b
, and erythropoie-
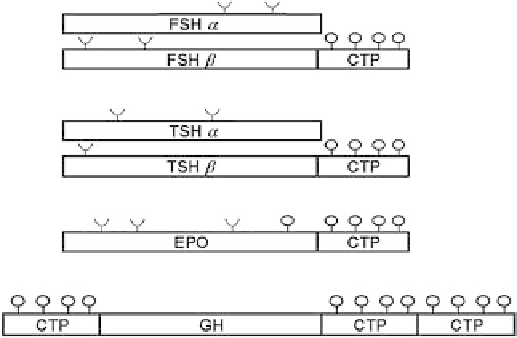
tin (EPO) (Figure 13.3). Crystallographic studies indicated
that both sides of human growth hormone (hGH): N-
terminal and C-terminal are not involved in receptor binding
and they are accessible (Figure 13.4). Therefore, CTP was
ligated to the N-terminal and to the C-terminal of hGH
coding sequence (Figure 13.3).
FIGURE 13.3
Construction of glycoprotein hormone chimeras
containing the translated sequence of FSH
b
-subunit, TSH
b
-
subunit, EPO, GH, and hCG
b
-carboxyl-terminal peptide. O-linked
oligosaccharides were indicates as
, and N-linked oligosacchar-
ides were indicated as
.









Search WWH ::

Custom Search