Biomedical Engineering Reference
In-Depth Information
compared with mice receiving the higher 24mg/kg dose of
HSA-IFN-
a
2b, in this case with dosing on days 6 and 13
post tumor implantation. In both groups, a statistically
significant reduction in tumor growth was observed in
comparison with the vehicle control group; however,
when compared directly, the differences between the two
groups were not statistically significant (P
0.0715). These
data demonstrate the improved in vivo efficacy of IFN-
a
2b-
DOM7h-14 compared with HSA-IFN-
a
2b in this model.
When dosed at molar equivalence, a significantly greater
reduction in tumor growth is observed in the IFN-
a
2b-
DOM7h-14 group. When IFN-
a
2b-DOM7h-14 is adminis-
tered at a 10-fold lower dose than HSA-IFN-
a
2b, the level of
reduction in tumor growth is indistinguishable between the
two groups, demonstrating that even with drastically
reduced doses, in comparison to the HSA fusion protein,
the AlbudAb fusion protein maintains comparable efficacy
in preclinical animal models.
Using IFN as a showcase, we have demonstrated the
utility of the AlbudAb platform in creating novel long acting
therapeutics with improved in vitro potency, in vivo half-
lives, and preclinical efficacy in animal models, compared to
clinically validated half-life extension technologies such as
PEGylation and HSA fusion. This novel method of half-life
extension potentially addresses issues of heterogeneity asso-
ciated with PEGylation and can be applied to a wide variety
of expression systems, unlike serum albumin fusion proteins
which typically require yeast expression systems; however,
it should be noted that although AlbudAbs themselves are
amenable to expression in bacterial, yeast, and mammalian
hosts, the therapeutic molecule to which the dAb is fused
may also govern the choice of expression host used to
manufacture the AlbudAb fusion protein.
It remains to be seen whether our IFN-AlbudAb fusion
proteins exhibit the same heterogeneity and instability dur-
ing long-term storage and formulation for therapeutic deliv-
ery as is seen with some HSA-therapeutic fusions, though
data collected to date have shown that these IFN-AlbudAb
molecules exhibit the same desirable properties (monomeric
solution state, thermal stability, lack of proteolytic cleavage,
and heterogeneity following purification) observed with
many isolated dAb molecules characterized to date (our
unpublished data).
¼
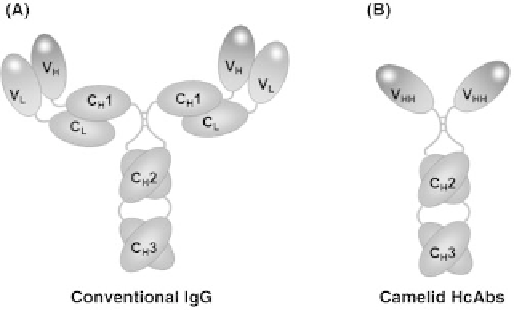
FIGURE 11.6
Structure of conventional immunoglobulins and
camelid heavy chain antibodies. Schematic diagram showing
domain structure of conventional IgG (A), compared with camelid
HcAbs (B). Camelid HcAbs are devoid of any light chain interac-
tion, and possess no C
H
1 domain in the heavy chain, with the V
HH
variable domain fused directly to the C
H
2 hinge region.
composed of two identical heavy chain polypeptides in
the complete absence of any discernible light chain
[28,29]. Referred to as heavy chain antibodies (HcAbs,
Figure 11.6), this alternative format of immunoglobulin is
found in addition to the classical immunoglobulin (i.e.,
heavy and light chain containing) format and bears an
extensive antigen-binding repertoire because of the presence
of a single variable region termed the V
HH
domain, which
retains antigen-binding activity when cloned and expressed
as a single domain from E. coli [30]. V
HH
domains bear a
number of structural similarities with single variable
domains from classical immunoglobulin, and this type of
single domain antibody is being developed by Ablynx as
nanobody technology for similar clinical and commercial
applications as those currently being targeted by dAbs.
Although dAbs and nanobodies share many common
features, for example, a structure consisting of four
constant “framework regions” and three hypervariable
“complementarity determining regions” (CDRs numbered
CDR1, 2, and 3) responsible for antigen specificity as well as
the same immunoglobulin fold composed of two
b
-sheets
[31], the two types of molecule also differ in several minor
but important respects. For example, the CDR1 and CDR3
regions of nanobodies are longer than those found in V
H
dAbs. In some cases, CDR1 and CDR3 also contain a
cysteine residue, which participates in disulfide bond for-
mation and may be involved in maintaining tertiary structure
[32]. The framework 2 region of nanobodies and V
H
dAbs
also exhibits major differences in primary amino acid
sequence between the two molecules. In V
H
dAbs, this
region is composed primarily of residues, which form
part of the interface between V
L
and V
H
domains in the
complete immunoglobulin structure. However, framework 2
is composed predominantly of different residues in V
HH
11.4 NANOBODIES IN THE DEVELOPMENT
OF ALTERNATIVE HALF-LIFE EXTENSION
TECHNOLOGIES BASED ON SINGLE
IMMUNOGLOBULIN VARIABLE DOMAINS
Immunoglobulins from the majority of mammalian species
consist of two identical heavy and light chain polypeptides.
Many species of camelidae, including camels and llamas,
have an alternative type of immunoglobulin, which is
Search WWH ::

Custom Search