Biomedical Engineering Reference
In-Depth Information
transplanted into mice with a severe combined immuno-
deficiency due to a recessive mutation in the Prkdc gene
(CB17/ICR-Prkdcscid/IcrCrl mice, hereafter referred to as
SCID mice). Owing to the lack of a humoral or cellular
immune response in these mice, human tumor cell lines
could be “xenografted” onto the right or left flank where they
form distinct tumor masses [26]. Retardation of tumor
growth in response to treatment with human IFN-
a
2b fusion
proteins can be determined as a measure of the in vivo
efficacy of these molecules, and in these experiments,
female SCID mice were xenografted with the 518A2 human
melanoma cell line, which previous investigators have
shown to be sensitive to human IFN-
a
following transplan-
tation in this manner [27]. Subcutaneous injections of
vehicle control (PBS), or equimolar amounts of either
IFN-
a
2b-DOM7h-14 (8.75 mg/kg) or HSA-IFN-
a
2b
(24mg/kg) were administered on days 1 and 8 post tumor
implantation (Figure 11.5). Both human IFN-
a
2b fusion
proteins were able to significantly reduce the rate of increase
in tumor volume as shown by a comparison of the measure-
ments obtained from these two groups with those obtained
from the vehicle control group; however, the reduction
observed with the IFN-
a
2b-DOM7h-14 group was greater
than that observed in the HSA-IFN-
a
2b group, consistent
with the improved in vitro potency and in vivo half-life of
IFN-
a
2b-DOM7h-14 compared with that of HSA-IFN-
a
2b.
In order to determine whether these results were statistically
significant, slopes of the graphs obtained up to day 34 were
compared using random coefficient regression analysis, and
the reduction in tumor growth rates in the two treatment
groups was shown to be significantly different (P
TABLE 11.2 Pharmacokinetics of Interferon Fusion
Proteins Following Subcutaneous Administration in Rats
IFN-
a
2b-
DOM7h-14
HSA-IFN-
a
2b
Unfused
IFN-
a
T
1/2
elim (h)
28.3
19.7
1.5
C
max
(
m
g/mL)
5.9
2.9
1.39
AUC
0-
1
342.5
137.1
5.5
Clearance (mL/h/kg)
5.9
16.7
357.1
Bioavailability (%)
47.7
19.9
39.9
Source: Reproduced from Reference [20] by permission of Oxford Univer-
sity Press.
compared with HSA-IFN-
a
2b (46.4% vs. 19.9%) with the
reason for this difference currently undetermined.
In summary, we have demonstrated in rodent pharmaco-
kinetic assays that AlbudAb technology can be used to
generate fusion proteins with improved pharmacokinetics
compared with genetic serum albumin fusions. The reason
for this is currently unclear but may be due in part to the
difference in interactions with FcRn of the two fusion
proteins. It is possible that direct fusion of therapeutic
proteins to HSA adversely affects binding of the albumin
fusion partner to FcRn, whereas AlbudAb binding to HSA
disrupts the albumin-FcRn interaction to a lesser degree,
though this remains to be experimentally determined.
11.3.3 In Vivo Efficacy of HSA and AlbudAb Fusion
Proteins
In order to compare the in vivo efficacy of human IFN-
a
2b
AlbudAb and HSA fusion proteins, we used a model in
which human tumor cell lines sensitive to type I IFNs were
0.001).
The effect of a 10-fold lower dose (0.875 mg/kg) of IFN-
a
2b-DOM7h-14 in this model was also investigated and
¼
1800
1600
1400
1200
1000
800
600
400
200
0
= dose
048 2 6 0
24
28
32
36
40
44
Days post implant
FIGURE 11.5
In vivo efficacy of IFN-
a
2b fusion proteins in A518A2 xenograft model. Tumor
volume in A518A2 xenografted mice injected with 8.75mg/kg IFN-
a
2b-DOM7h-14 (- - -
&
- - -),
24mg/kg HSA-IFN-
a
2b (-
~
-), or PBS (-
-) at days 1 and 8 post tumor implant. Tumor volume was
measured at the indicated times, with n
¼
10 at each time point (error bars shown represent s.e.m. at
each time point). Statistical comparison of the tumor volumes in the IFN-
a
2b-DOM7h-14 and HSA-
IFN-
a
2b dose groups was carried out using linear regression coefficient analysis and differences
found to be significant (P
¼
0.0001).
Source: Reproduced from Reference [20] by permission of Oxford
University Press.







































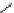













































































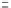
























Search WWH ::

Custom Search