Biomedical Engineering Reference
In-Depth Information
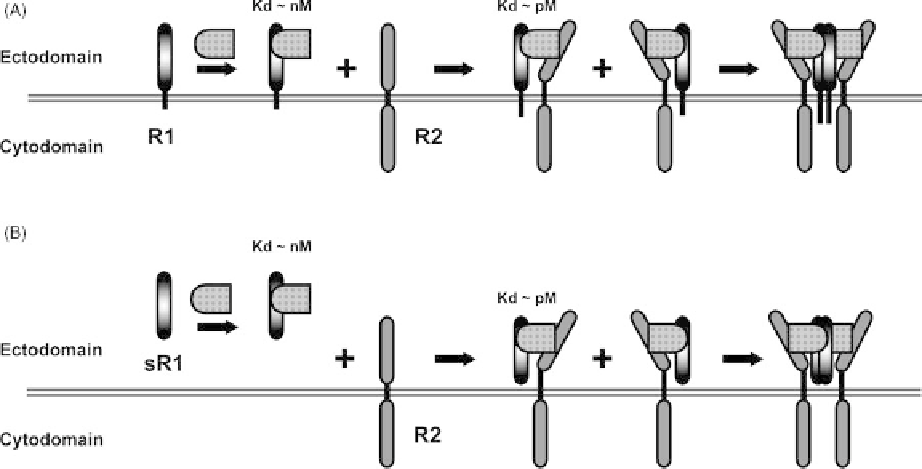
FIGURE 9.3
Trans-signaling. (A) Schematic representation of a receptor system utilizing a
specificity-determining (but nonsignaling) receptor (R1). R1 is tethered to the cell membrane either
by a transmembrane domain followed by a short cytoplasmic tail as shown here (e.g., IL-6R, IL-11R),
or by a GPI anchor (e.g., CNTFR, GFRA1, GFRA2). Mirroring other multicomponent receptor
systems (see Figure 9.1), cognate ligands are first recognized by R1. Binding of ligand to R1 results in
the formation of a low-affinity R1
ligand complex. The R1
ligand complex engages a second (usually
shared or “common”) receptor subunit to form a high-affinity R1
ligand
R2. Unlike the multi-
component receptor systems shown on Figure 9.1, this R1
ligand
R2 complex does not transduce
signal; a third step involving dimerization of R1
ligand
R2 is required to initiate signaling. (B) Cells
that do not express R1 are not responsive to ligand. However, soluble receptor 1 (sR1) can present
ligand to these cells and initiate the same cascade of events depicted in the upper panel. This process
has been termed trans-signaling.
subunit (e.g., IL-6R for IL-6; CNTFR for CNTF; IL-
11RA for IL-11, GDNF family receptor
a
-1 or -2
(GFRA1 or GFRA2) for glial cell-derived neurotro-
phic factor, (GDNF)) to generate a receptor 1
engineering problem, that is, how to put together receptor
ectodomains in a manner such that resulting hybrid proteins
will function as high-affinity ligand traps. In addition, these
hybrids should demonstrate high level of expression, easy
purification, and desirable pharmacokinetic profiles, which
will enable their development into therapeutics.
Generating heterodimers of receptor ectodomains in a
way that mirrors their configuration on the cell surface posed
a formidable technical problem. It was also not known
whether such complexes would act as effective and specific
ligand traps. In order to obtain rapid proof-of-concept,
heteromeric ligand traps were generated for two members
of the IL-6 family of cytokines—CNTF and IL-6—by
engineering ectodomain heterodimers between IL-6R and
the shared receptor chain gp130 for IL-6 and CNTFR and
gp130 for CNTF, respectively (Economides and Stahl,
unpublished results). In a first iteration, the ectodomains
of IL-6R or CNTFR and gp130 were fused to leucine zippers
derived from Fos and Jun, as they assemble into hetero-
dimers [129]. This strategy was successful in generating IL-
6R:gp130 and CNTFR:gp130 ectodomain heterodimers that
ligand
complex, followed by binding of a second and some-
times a third receptor subunit, which are usually
shared [117,124,125,128].
2. In this stepwise complex formation, the second step is
also an “affinity conversion” step, because the result-
ing complex—receptor 1
receptor 2—is much
more stable than the one resulting from the initial
association of the ligand with receptor 1 [117,124].
ligand
These observations led to the hypothesis that heteromeric
hybrid proteins composed of the ectodomains of receptor 1
(R1) and receptor 2 (R2) should form nonsignaling com-
plexes with their cognate ligands and display the high
affinity observed with their natural counterparts. Further-
more, for ligands that share a signaling coreceptor, the
specificity of the Trap should be determined by the choice
of R1. These hypotheses were then reduced to an
Search WWH ::

Custom Search