Biomedical Engineering Reference
In-Depth Information
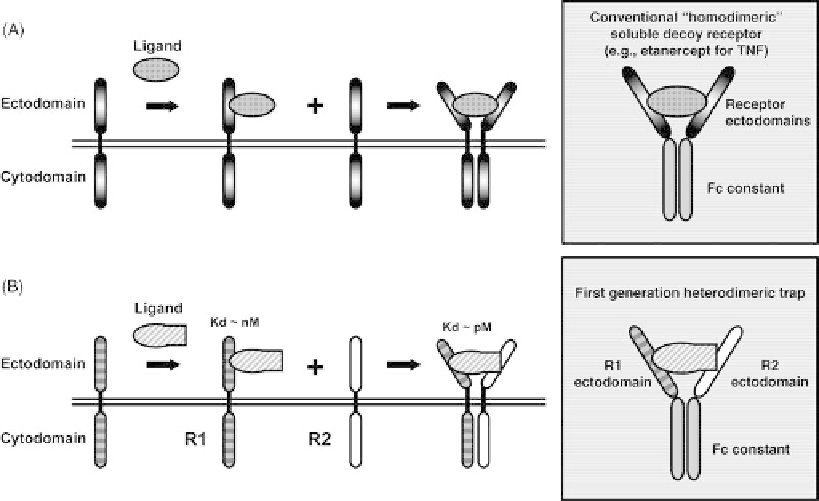
FIGURE 9.1
First-generation Traps for ligands utilizing multicomponent receptor systems.
(A) Schematic representation of a ligand-receptor system that utilizes a single receptor. Binding
of ligand to a first receptor subunit is followed by dimerization (or multimerization) with another
identical subunit and formation of a signaling complex. Although the exact stoichiometry of the
number of ligand and receptor monomers that comprise the signaling complex varies depending on
the receptor system, simple dimerization of the receptor ectodomains via Fc results in a receptor-Fc-
based blocker for the cognate ligand (upper inset). An example of this is etanercept, a receptor-Fc
fusion protein composed of TNFR-Fc that acts as a blocker of TNF [46]. (B) Schematic representa-
tion of a ligand-receptor system that utilizes a two-component receptor, where the second receptor
(R2) may be shared among different members of a family of ligands. Binding of ligand to the first
receptor subunit (R1) results in the formation of a low-affinity complex, with K
d
in the nanomolar
range. This step is required for recognition of the ligand and frequently imparts selectivity, as it is the
presence of a specific R1 that determines whether a cell will be responsive to a specific ligand. This
first step is followed by engagement of R2 by the R1
ligand complex, resulting in a high affinity,
signaling complex composed of R1
R2. Although the exact stoichiometry of the number of
ligand molecules and each of the two different receptor subunits that comprise the signaling complex
may vary, artificial heterodimerization of the ectodomains of these two receptor subunits, via the Fc
region of IgG, results in a high-affinity ligand trap (lower inset) with low picomolar affinity for its
cognate ligands. Examples of such heterodimeric receptor ectodomains-Fc fusion proteins are
presented by early versions of Cytokine Traps [53].
ligand
multiplicity of other cytokines [117]. These systems
may also incorporate additional accessory coreceptors
that facilitate binding but are otherwise dispensable,
that is, are not obligatory for recognition of ligand or
signal transduction [29,30,118-122].
2. Receptor Systems that Utilize Specificity-Determining
Nonsignaling Subunits (Figures 9.1b and 9.3). These
receptor systems employ shared or “common” sub-
units that are utilized by multiple ligands in any given
family, but which neither bind their ligand partners at
an appreciable level, nor do they confer specificity.
Examples of shared subunits are the common
g
chain
of the IL-2 family of cytokines (IL-2RG), and gp130
(also known as IL-6ST) for the IL-6 family of cyto-
kines [117]. Consequently, in these systems, binding
of ligand is conferred by specificity-determining sub-
units; this is an obligate step, required to initiate
formation of the signaling complex. Examples of
such receptor subunits are IL-6R, CNTFR, IL-
11RA, GFRA1, and GFRA2 [117,123-125]. These
specificity-determining subunits are not capable of
signaling either because they are tethered to the
membrane by a glycosylphosphatidylinositol (GPI)
anchor or because their cytoplasmic domains lack
enzymatic activity or binding sites for accessory
molecules.
Search WWH ::

Custom Search