Biomedical Engineering Reference
In-Depth Information
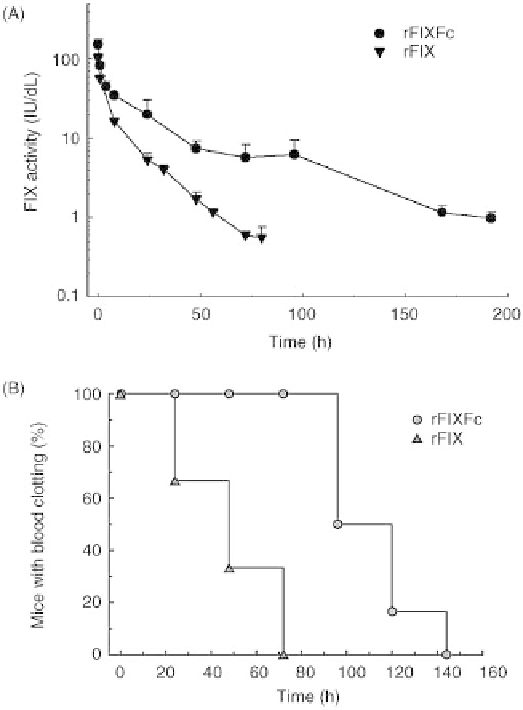
FIGURE 7.4
Functional activities of rFIXFc and rFIX in FIX-deficient mice. (a) Clotting activity
measured by modified aPTT assay in FIX-deficient mice. FIX-deficient mice were administered a
single intravenous dose of rFIXFc (200 IU/kg, n
¼
4 mice per time point). Blood samples were
collected at predetermined time points and analyzed for FIX activity (mean
SD). (b) FIX-deficient
mice (six per group) were dosed intravenously with 50 IU/kg rFIXFc or rFIX. Blood samples were
collected before dosing and at various times after dosing. Blood samples were incubated at 37
C and
were visually inspected for the presence of a blood clot once per minute. The time needed for a clot to
form was recorded, and, once the clotting activity returned to baseline (i.e., no clot formation), no
additional samples were obtained (samples were collected 15min to 144 h for rFIXFc or 15min to
72 h for rFIX). Source (b): This research was originally published in Reference [61].
admin istered (1 and 5 IU/ k g, one su bj ect in each group ) were
assessed for safety o nly. In additio n to s afe ty, the FIX activity
PK parameters were assessed in patients, wh o rec eived highe r
rFIXFc dose l evels (1 2.5, 2 5, 50, an d 100 IU/ k g). D ose-pro -
portio nal in crease i n plasma FIX activity wa s observe d based
on C
max
o ccurrin g immediately after infusion and to tal expo -
sure (AUC
INF
) (Fig ure 7.5 ). Mean activity terminal half-life fo r
rFIXFc was 5 6.7 h, appro ximately th reefold l on ger tha n his -
to rical values reported f or rFIX [65-6 7] . T he in cr emen tal
recove ry of rF IXFc was 0.93 IU/ dL per IU/ kg, an improvemen t
compared with rFIX and similar to p las ma-derived FIX prod -
ucts [65- 67]. These results show that rFIXFc may offer a v iab le
th erapeutic approach to achieve prolonged hemostatic p ro tec -
tion an d less freque nt do sin g in p atients with hemoph ilia B.
Th erefo re, the P K p arameters d etermined from p rec linical
studies in several different animal mo dels compared well
with the PKs of rFIXFc determined in humans. The results
from the Phase I/IIa rFIXFc study supported development of
the Phase III study to evaluate the safety, PKs, and efficacy of
rFIXFc in previously treated patients with severe hemophilia B
(www.clinicaltrials.gov iden ti fier N CT 0102 7364 ).
7.4 ALTERNATIVE APPLICATIONS
Fc monomers often have improved bioactivity and PK
parameters compared to dimeric counterparts when the
effector molecule is naturally a monomer. The concept of
Search WWH ::

Custom Search