Biomedical Engineering Reference
In-Depth Information
occurring within the living organisms (including Krebs cycle) were applied by El Fray et al. as
nontoxic cross-linking agents [35-37]. Due to the presence of two or more functional groups (car-
boxylic groups, hydroxyl groups), hydrogen bonds are additionally formed in the polymer network
during freezing-thawing process, leading to cryogels with mechanical properties useful for car-
tilage repair. In applied freezing-thawing process, water acts as an expanding agent (porophor).
When the PVA solution is subjected to freezing, the pure solvent crystallizes initially, while the
solute stays in the liquid part of the specimen. Such a structure leads to stronger polymer-polymer
and polymer-carboxylic acid interactions that result in stable 3D cryogel network [38]. The reac-
tions proposed to occur between PVA and acids are presented in Figure 21.6.
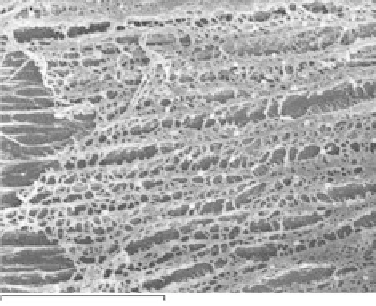
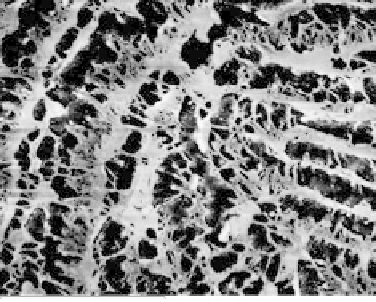
The internal porosity of cryogels reveals signifi cant correlation among number of freezing-
thawing cycles, the chemical structure of additive, and the pore size and arrangement. PVA cryogel
modifi ed with succinic acid after nine freezing-thawing cycles shows highly porous structure
with the pore size in the range of 1-2 µm (Figure 21.7a). The size of pores corresponds to the one
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
OH
OH
OH
OH
O
C
CH
2
CH
2
C
O
OH
OH
O
C
CH
2
CH
2
C
O
O
O
OH
O
OH
H
O
O
OH
OH
OH
OH
O
O
CH
CH
2
CH
CH
2
CH
2
CH
OH
OH
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
(a)
(b)
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
OH
O
O
OH
O
C
C
H
2
H
2
C
O
OH
OH
OH
OH
CH
2
CH
CH
2
CH
CH
2
CH
CH
2
CH
(c)
FIGURE 21.6
Scheme of possible reactions occurring between PVA chain and gluconic (a) and succinic
acids (b and c).
10
µ
m
(a)
(b)
FIGURE 21.7
Internal porosity of PVA cryogel modifi ed with (a) succinic acid and (b) gluconic acid.