Biomedical Engineering Reference
In-Depth Information
Eudragit (dissolved in a solvent consisting of acetone: ethanol) to produce an organic phase, which
was then poured into liquid paraffi n containing sorbitan trioleate (Span 85) and a foam suppressor
(Antifoam A). The system was maintained under agitation for 3 h at room temperature to allow
evaporation of the solvent. The encapsulated microspheres were collected, rinsed with
n-
hexane,
and freeze-dried. Drug delivery from the microspheres was reported to occur after pH-dependent
dissolution of the Eudagrit coating (the duration of which depended on the type of Eudagrit used),
swelling of the chitosan microspheres, and dissolution of the model drug [62].
Mucoadhesive polymers offer increased drug bioavailability at target mucosal tissues without
dilution or degradation in lumenal fl uids. Prerequisites for a good mucoadhesive polymer include high
fl exibility of its polymer backbone structure and of its polar functional groups. Suggested mechanisms
that may underlie the mucoadhesion between biomaterials and mucin include electrostatic adsorp-
tion (van der Waals, hydrogen bonds), wetting, diffusion, and fracture theories [64]. When hydrated,
chitosan demonstrates good mucoadhesive properties through its interaction with mucin [65].
20.6.1.2
Alginate-Based Drug Delivery Systems
Another mucoadhesive polymer is alginate, a negatively charged polysaccharide derived from brown
seaweed that is commonly used for the production of drug-loaded microparticles [66]. Because
alginate beads are nontoxic orally and have high biocompatibility, they have been developed for
use as controlled delivery devices to the colon for a number of different drugs [64,67,68]. Alginates
undergo reversible gelation (coacervation) in the presence of divalent cations, such as Ca
2
+
, to form
hydrogels [69]. Large beads (1-2 mm in diameter) can be readily produced by manually dropping
an alginate solution from a syringe into a solution of calcium chloride. Fabrication of smaller
beads down to about 200 µm in diameter can be achieved using a high electrostatic potential
bead generator (Figure 20.6). This instrument uses an electrostatic potential to pull alginate drop-
lets from a needle tip into a bath containing gelling ions, such as calcium chloride (Figure 20.7).
Several parameters can be used to control the fi nal size of the alginate beads produced and also the
effi ciency of drug entrapment. These parameters include the applied electrostatic potential, fl ow
rate of polymer solution, needle diameter, gelling ion concentration, hardening times, and alginate
composition, concentration, and viscosity [70,71].
Excellent mucoadhesive properties of alginate/chitosan beads to freshly excised pig intestine
has been reported [64]. The beads, intended for colonic drug delivery, were prepared by complex
coacervation using sodium alginate as a gel core. Sodium alginate (2% w/v) prepared in deionized
water was dropped through a 0.45 mm syringe needle (1 mL/min) into a solution of calcium chloride
(0.5-1.0% w/v) mixed with chitosan (0.5-1.5% w/v). The beads were allowed to harden for at least
500
µ
m
(a)
(b)
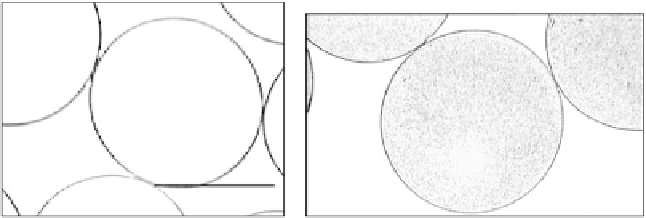
FIGURE 20.6
Alginate microspheres measuring approximately 600 µm in diameter containing (a) 0% or
(b) 0.1% (w/v) 45S5 bioactive glass produced using an electrostatic bead generator. Alginate solution (1.5%
w/v low viscosity) was delivered from a nozzle (outer diameter of 0.35 mm/inner diameter of 0.17 mm) at a
fl ow rate of 9 mL/h and electrostatic potential of 3.6 kV into a gelling solution of 15 mM CaCl
2
.