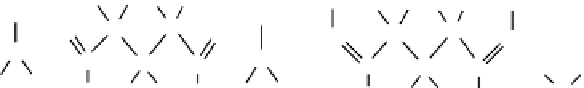Biomedical Engineering Reference
In-Depth Information
20.4.2.2
Collagen Bulking Materials
Collagen-based biomaterials have been evaluated for numerous medical applications. Contigen (CR
Bard, Covington, Georgia), derived from bovine collagen, was the fi rst approved injectable bulking
agent on the U.S. market and has been extensively studied for urinary incontinence. Contigen is
fabricated from dermal type I collagen that has been purifi ed, cross-linked with glutaraldehyde
solution, and dispersed in phosphate buffer saline. After injection, the collagen condenses into a
fi brous network that is colonized by host connective tissue cells and vasculature, which gradually
remodel and breakdown the implant. More recently collagen-based materials have been shown to
be effective for use in the treatment of idiopathic fecal incontinence in which the benefi cial effect
appears to result from the mechanical effect of bulking the tissue with collagen, thus preventing
fecal leakage [34].
Collagen-based biomaterials can be derived from bovine or porcine skin and porcine small intes-
tinal submucosa or bladder. To prepare dermal-derived collagen-based materials, the fat and epider-
mis are fi rst removed from the harvested hide, and then the dermis is soaked in detergent solutions.
Povidone-iodine and hydrogen chloride solutions are used to remove microbial contaminants that
may be present in the dermis, followed by hydroxide solutions to remove cellular debris and inactivate
prions and any remaining viruses. Gamma irradiation is used to further sterilize the tissue [35].
Collagen used in biomaterials is usually cross-linked to improve the mechanical stability, immu-
nogenic properties, and controlled biodegradability for long-term biomedical applications [36].
The predominant chemical treatment for collagen-based biomaterials has been cross-linking with
glutaraldehyde, because it is inexpensive, readily available, and highly soluble in aqueous solution,
resulting in a biomaterial that is less susceptible to biodegradation. The precise chemistry underlying
the cross-linking of collagen by glutaraldehyde is not certain, but it is suggested that glutaraldehyde
reacts with primary amines, cross-linking them in an inter- and intramolecular fashion via the
formation of covalent bonds (Figure 20.4). This cross-linking occurs either by the formation of unstable
Schiff base linkages through reaction of an aldehyde group with an amino group of lysine or hydroxy-
lysine or by the formation of stable aldol condensation products between two adjacent aldehydes [37].
However, concerns have been raised regarding the use of glutaraldehyde cross-linked bioma-
terials since unstable glutaraldehyde polymers that can remain in the interstices of cross-linked
bioprostheses may slowly leach out, causing prolonged local cytotoxicity [38]. Although the cyto-
toxic effects of glutaraldehyde cross-linked collagen can be decreased by extensive rinsing or by the
addition of quenching reagents, such as propylhydroxybenzoate, methylhydroxybenzoate, and gly-
cine [39], alternative cross-linking agents have been proposed. These include dicyclohexylmethane-
4,4-diisocyanate (HMDI), which cross-links three amino acids in triple helix with hexamethylene
diisocyanate. It is suggested that HMDI cross-linking produces fewer cytotoxic effects compared
with those caused by glutaraldehyde cross-linking, where aldehyde leaching from the materials may
cause an infl ammatory response [35].
Although collagen is a naturally occurring biomaterial, the chemical processing it requires
before it can be used as a long-term implant leads to a number of drawbacks. For example, although
cross-linking slows down the rate of biodegradation of the collagen implant by cellular proteases,
there is also a possibility that cross-linking may prevent cellular infi ltration into the biomaterial
from host tissue [35]. Furthermore, calcifi cation of bioprostheses fabricated from tissue-derived
Glutaraldehyde
Protein
Protein
HHHH
R
H
HH H
R
R
R
+
C
C
O
O
+
C
C
N
N
N
N
C
C
C
C
C
H
H
C
+
HH
O
H
HH
H
HH
(
×
2)
HHHH
FIGURE 20.4
Cross-linking reaction of glutaraldehyde with primary amines on protein molecules.