Biomedical Engineering Reference
In-Depth Information
Cell and tissue remodeling is important for achieving stable biomechanical conditions and vas-
cularization at the host site. Hence, the 3-D scaffold/tissue construct should maintain suffi cient
structural integrity during the
in vitro
and
in vivo
growth and remodeling process. The degree of
remodeling depends on the tissue itself (e.g., skin 4-6 weeks, bone 4-6 months) and its host anatomy
and physiology. Scaffold architecture has to allow for initial cell attachment and subsequent migra-
tion into and through the interconnecting pore volume, mass transfer of nutrients and metabolites,
provision of suffi cient space for development, and later remodeling of organized tissue. Figure 2.2
shows schematically the process of cell attachment till mineralization for a scaffold intended for
bone engineering application.
The degradation and resorption kinetics of the scaffold need to be designed based on the rela-
tionships of mechanical properties, molecular weight (
M
w
/
M
n
), mass loss, and tissue development.
A number of studies
12-15
have demonstrated the dependence of mechanical properties on scaffold
porosity when designing and fabricating scaffolds via solid free-form fabrication (SFF). Logi-
cally, as porosity of the scaffold is increased (decline in material bulk) its mechanical properties
would decrease correspondingly. This decline has been found to follow a power-law relationship
(Figure 2.3).
In addition to these essentials of mechanics and geometry, a suitable construct will possess sur-
face properties that are optimized for the attachment and migration of cell types of interest (depend-
ing on the targeted tissue). The external size and shape of the construct must also be considered,
especially if the construct is to be customized for an individual patient.
2
A number of fabrication technologies have been applied to process biodegradable and biore-
sorbable materials into 3-D polymeric scaffolds of high porosity and surface area.
16
From a scaffold
100
µ
m
B
Cell attachment
Cell spreading
A
200
µ
m
100
µ
m
Cell bridging
C
100
m
µ
100
m
µ
Cell networking
Cell mineralization
D
E
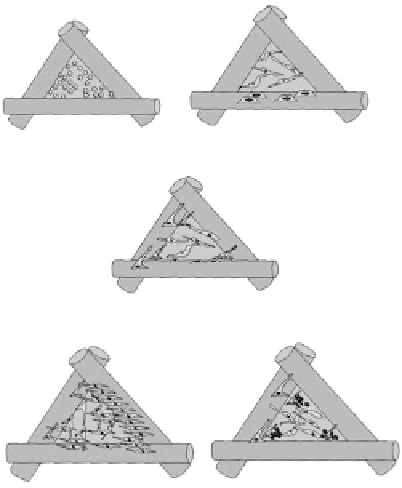
FIGURE 2.2
Sequences of scaffold neo-tissue formation,
in vitro
. Cells manually (pipetted) or dynamically
(using a bioreactor) seeded and attach to the scaffold surface (A). Seeding effi ciency is highly dependent upon
the surface itself but also on the surface to volume ratio of the scaffold. Attachment is followed by cell spreading
(B) and bridging across the scaffold pores (C), cell networking then follows (D) with subsequent cell minerali-
sation (E) providing a fi rm basis for successful transferral and implantation of the neo-tissue construct
in vivo
.